The coronavirus transmissible gastroenteritis virus causes infection after receptor-mediated endocytosis and acid-dependent fusion with an intracellular compartment
- PMID: 9420255
- PMCID: PMC109404
- DOI: 10.1128/JVI.72.1.527-534.1998
The coronavirus transmissible gastroenteritis virus causes infection after receptor-mediated endocytosis and acid-dependent fusion with an intracellular compartment
Abstract
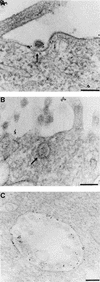
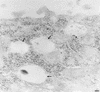
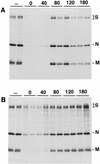
Aminopeptidase N is a species-specific receptor for transmissible gastroenteritis virus (TGEV), which infects piglets, and for the 229E virus, which infects humans. It is not known whether these coronaviruses are endocytosed before fusion with a membrane of the target cell, causing a productive infection, or whether they fuse directly with the plasma membrane. We have studied the interaction between TGEV and a cell line (MDCK) stably expressing recombinant pig aminopeptidase N (pAPN). By electron microscopy and flow cytometry, TGEV was found to be associated with the plasma membrane after adsorption to the pAPN-MDCK cells. TGEV was also observed in endocytic pits and apical vesicles after 3 to 10 min of incubation at 38 degrees C. The number of pits and apical vesicles was increased by the TGEV incubation, indicating an increase in endocytosis. After 10 min of incubation, a distinct TGEV-pAPN-containing population of large intracellular vesicles, morphologically compatible with endosomes, was found. A higher density of pAPN receptors was observed in the pits beneath the virus particles than in the surrounding plasma membrane, indicating that TGEV recruits pAPN receptors before endocytosis. Ammonium chloride and bafilomycin A1 markedly inhibited the TGEV infection as judged from virus production and protein biosynthesis analyses but did so only when added early in the course of the infection, i.e., about 1 h after the start of endocytosis. Together our results point to an acid intracellular compartment as the site of fusion for TGEV.
Figures



Similar articles
-
CD163 and pAPN double-knockout pigs are resistant to PRRSV and TGEV and exhibit decreased susceptibility to PDCoV while maintaining normal production performance.Elife. 2020 Sep 2;9:e57132. doi: 10.7554/eLife.57132. Elife. 2020. PMID: 32876563 Free PMC article.
-
Production of porcine aminopeptidase N (pAPN) site-specific edited pigs.Anim Sci J. 2019 Mar;90(3):366-371. doi: 10.1111/asj.13163. Epub 2019 Jan 8. Anim Sci J. 2019. PMID: 30623527 Free PMC article.
-
Porcine Deltacoronavirus Engages the Transmissible Gastroenteritis Virus Functional Receptor Porcine Aminopeptidase N for Infectious Cellular Entry.J Virol. 2018 May 29;92(12):e00318-18. doi: 10.1128/JVI.00318-18. Print 2018 Jun 15. J Virol. 2018. PMID: 29618640 Free PMC article.
-
Transmissible gastroenteritis virus infection: a vanishing specter.Dtsch Tierarztl Wochenschr. 2006 Apr;113(4):157-9. Dtsch Tierarztl Wochenschr. 2006. PMID: 16716052 Review.
-
Early interactions between animal viruses and the host cell: relevance to viral vaccines.Vaccine. 1986 Jun;4(2):79-90. doi: 10.1016/0264-410x(86)90042-3. Vaccine. 1986. PMID: 3014773 Free PMC article. Review.
Cited by
-
CD13 mediates phagocytosis in human monocytic cells.J Leukoc Biol. 2015 Jul;98(1):85-98. doi: 10.1189/jlb.2A0914-458R. Epub 2015 May 1. J Leukoc Biol. 2015. PMID: 25934926 Free PMC article.
-
SARS-CoV-2 Evolutionary Adaptation toward Host Entry and Recognition of Receptor O-Acetyl Sialylation in Virus-Host Interaction.Int J Mol Sci. 2020 Jun 26;21(12):4549. doi: 10.3390/ijms21124549. Int J Mol Sci. 2020. PMID: 32604730 Free PMC article. Review.
-
Myricetin inhibits transmissible gastroenteritis virus replication by targeting papain-like protease deubiquitinating enzyme activity.Front Microbiol. 2024 Jul 10;15:1433664. doi: 10.3389/fmicb.2024.1433664. eCollection 2024. Front Microbiol. 2024. PMID: 39050632 Free PMC article.
-
CD13/APN regulates endothelial invasion and filopodia formation.Blood. 2007 Jul 1;110(1):142-50. doi: 10.1182/blood-2006-02-002931. Epub 2007 Mar 15. Blood. 2007. PMID: 17363739 Free PMC article.
-
Recently discovered human coronaviruses.Clin Lab Med. 2009 Dec;29(4):715-24. doi: 10.1016/j.cll.2009.07.007. Clin Lab Med. 2009. PMID: 19892230 Free PMC article. Review.
References
-
- Asanaka M, Lai M C. Cell fusion studies identified multiple cellular factors involved in mouse hepatitis virus entry. Virology. 1993;197:732–741. - PubMed
-
- Bretscher M S. Endocytosis: relation to capping and cell locomotion. Science. 1984;224:681–686. - PubMed
-
- Davis C G, Goldstein J L, Südhof T C, Anderson R G W, Russell D W, Brown M S. Acid-dependent ligand dissociation and recycling of LDL receptor mediated by growth factor homology region. Nature. 1987;326:760–765. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

