An arenavirus RING (zinc-binding) protein binds the oncoprotein promyelocyte leukemia protein (PML) and relocates PML nuclear bodies to the cytoplasm
- PMID: 9420283
- PMCID: PMC109432
- DOI: 10.1128/JVI.72.1.758-766.1998
An arenavirus RING (zinc-binding) protein binds the oncoprotein promyelocyte leukemia protein (PML) and relocates PML nuclear bodies to the cytoplasm
Abstract
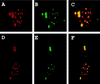
The promyelocytic leukemia protein (PML) forms nuclear bodies which are altered in some disease conditions. We report that the cytoplasmic RNA virus lymphocytic choriomeningitis virus (LCMV) influences the distribution of PML bodies. In cells infected with LCMV, the Z protein and PML form large bodies primarily in the cytoplasm. Transient transfection studies indicate that Z alone is sufficient to redistribute PML to the cytoplasm and that PML and Z colocalize. Coimmunoprecipitation studies show specific interaction between PML and Z proteins. A similar result was observed with a Z protein from another arenavirus, Lassa virus, suggesting that this is a general feature of the Arenaviridae. Genetically engineered mutations in PML were used to show that the Z protein binds the N-terminal region of PML and does not need the PML RING or the nuclear localization signal to colocalize. The Z protein acts dominantly to overcome the diffuse phenotype observed in several PML mutants. The interaction between PML and Z may influence certain unique characteristics of arenavirus infection.
Figures






References
-
- Banerjee S N, Buchmeier M, Rawls W E. Requirement of a cell nucleus for the replication of an arenavirus. Intervirology. 1976;6:190–196. - PubMed
-
- Barlow P N, Luisi B, Milner A, Elliot M, Everett R. Structure of the C3HC4 domain by 1H-nuclear magnetic resonance spectroscopy. J Mol Biol. 1994;237:201–211. - PubMed
-
- Boddy M N, Howe K, Etkin L D, Solomon E, Freemont P S. PIC1, a novel ubiquitin-like protein which interacts with the PML component of a multiprotein complex that is disrupted in acute promyelocytic leukaemia. Oncogene. 1996;13:971–982. - PubMed
-
- Borden K L B, Freemont P S. The RING finger: an example of a sequence structure family. Curr Opin Struc Biol. 1996;6:395–401. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

