Role of the cold-box region in the 5' untranslated region of the cspA mRNA in its transient expression at low temperature in Escherichia coli
- PMID: 9422597
- PMCID: PMC106853
- DOI: 10.1128/JB.180.1.90-95.1998
Role of the cold-box region in the 5' untranslated region of the cspA mRNA in its transient expression at low temperature in Escherichia coli
Abstract
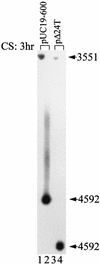
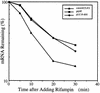
Upon temperature downshift, a group of proteins called cold shock proteins, such as CspA, CspB, and CsdA, are transiently induced in Escherichia coli. However, when the 5' untranslated region (5' UTR) of cspA mRNA is overproduced at low temperature, the expression of cold shock genes is prolonged or derepressed. It has been proposed that this effect is due to highly conserved 11-base sequences designated the "cold box" existing in the 5' UTRs of cspA, cspB, and csdA. Here, we demonstrate that the overproduction of the 5' UTR of not only cspA but also cspB and csdA mRNAs causes derepression of all three genes at the same time. Conversely, when the cold-box region was deleted from the cspA 5' UTR its derepression function was abolished. The amount of mRNA from the chromosomal cspA gene was much higher in cells overproducing the wild-type 5' UTR by means of a plasmid than it was in cells overproducing the cold-box-deleted 5' UTR. The stability of the chromosomal cspA mRNA in cells overproducing the wild-type 5' UTR was almost identical to that in cells overproducing the cold-box-deleted 5' UTR. Therefore, the derepression of cspA caused by overproduction of 5' UTR at the end of the acclimation phase occurs at the level of transcription but not by mRNA stabilization, indicating that the cold-box region plays a negative role in cspA transcription in cold shock-adapted cells. The role of the cold-box region was further confirmed with a cspA mutant strain containing a cold-box-deleted cspA gene integrated into the chromosome, which showed a high level of constitutive production of CspA but not CspB during exponential growth at low temperature.
Figures




Similar articles
-
The role of the 5'-end untranslated region of the mRNA for CspA, the major cold-shock protein of Escherichia coli, in cold-shock adaptation.J Bacteriol. 1996 Aug;178(16):4919-25. doi: 10.1128/jb.178.16.4919-4925.1996. J Bacteriol. 1996. PMID: 8759856 Free PMC article.
-
Low temperature regulated DEAD-box RNA helicase from the Antarctic archaeon, Methanococcoides burtonii.J Mol Biol. 2000 Mar 31;297(3):553-67. doi: 10.1006/jmbi.2000.3585. J Mol Biol. 2000. PMID: 10731411
-
Mutation analysis of the 5' untranslated region of the cold shock cspA mRNA of Escherichia coli.J Bacteriol. 1999 Oct;181(20):6284-91. doi: 10.1128/JB.181.20.6284-6291.1999. J Bacteriol. 1999. PMID: 10515916 Free PMC article.
-
The CspA family in Escherichia coli: multiple gene duplication for stress adaptation.Mol Microbiol. 1998 Jan;27(2):247-55. doi: 10.1046/j.1365-2958.1998.00683.x. Mol Microbiol. 1998. PMID: 9484881 Review.
-
The cold-shock response--a hot topic.Mol Microbiol. 1994 Mar;11(5):811-8. doi: 10.1111/j.1365-2958.1994.tb00359.x. Mol Microbiol. 1994. PMID: 8022259 Review.
Cited by
-
Changes in cspL, cspP, and cspC mRNA abundance as a function of cold shock and growth phase in Lactobacillus plantarum.J Bacteriol. 2000 Sep;182(18):5105-13. doi: 10.1128/JB.182.18.5105-5113.2000. J Bacteriol. 2000. PMID: 10960094 Free PMC article.
-
Recognition of T-rich single-stranded DNA by the cold shock protein Bs-CspB in solution.Nucleic Acids Res. 2006;34(16):4561-71. doi: 10.1093/nar/gkl376. Epub 2006 Sep 6. Nucleic Acids Res. 2006. PMID: 16956971 Free PMC article.
-
Selective mRNA degradation by polynucleotide phosphorylase in cold shock adaptation in Escherichia coli.J Bacteriol. 2001 May;183(9):2808-16. doi: 10.1128/JB.183.9.2808-2816.2001. J Bacteriol. 2001. PMID: 11292800 Free PMC article.
-
Cold shock induction of the cspL gene in Lactobacillus plantarum involves transcriptional regulation.J Bacteriol. 2002 Oct;184(19):5518-23. doi: 10.1128/JB.184.19.5518-5523.2002. J Bacteriol. 2002. PMID: 12218042 Free PMC article.
-
Importance of trmE for growth of the psychrophile Pseudomonas syringae at low temperatures.Appl Environ Microbiol. 2009 Jul;75(13):4419-26. doi: 10.1128/AEM.01523-08. Epub 2009 May 8. Appl Environ Microbiol. 2009. PMID: 19429554 Free PMC article.
References
-
- Belasco J G. mRNA degradation in prokaryotic cells: an overview. In: Belasco J G, Brewermann G, editors. Control of mRNA stability. New York, N.Y: Academic Press; 1993. pp. 3–11.
-
- Brandi A, Pietroni P, Gualerzi C O, Pon C L. Post-transcriptional regulation of CspA expression in Escherichia coli. Mol Microbiol. 1996;19:231–240. - PubMed
-
- Etchegaray J P, Jones P G, Inouye M. Differential thermoregulation of two highly homologous cold-shock genes, cspA and cspB of Escherichia coli. Genes Cells. 1996;1:171–178. - PubMed
-
- Fang L, Jiang W, Bae W, Inouye M. Promoter independent cold-shock induction of cspA and its derepression at 37°C by mRNA stabilization. Mol Microbiol. 1997;19:241–248. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

