Glycerol monolaurate inhibits induction of vancomycin resistance in Enterococcus faecalis
- PMID: 9422612
- PMCID: PMC106868
- DOI: 10.1128/JB.180.1.182-185.1998
Glycerol monolaurate inhibits induction of vancomycin resistance in Enterococcus faecalis
Abstract
Glycerol monolaurate (GML) is a surfactant that has been found to inhibit the post-exponential phase activation of virulence factor production and the induction of beta-lactamase in Staphylococcus aureus. It has been suggested that signal transduction is the most probable target for GML (S. J. Projan, S. Brown-Skrobot, P. M. Schlievert, F. Vandenesch, and R. P. Novick, J. Bacteriol. 176:4204-4209, 1994). We found that GML suppresses growth of vancomycin-resistant Enterococcus faecalis on plates with vancomycin and blocks the induction of vancomycin resistance, which involves a membrane-associated signal transduction mechanism, either at or before initiation of transcription. Given the surfactant nature of GML and the results of previous experiments, we suggest that GML blocks signal transduction. In contrast, GML has no effect on the induction of erythromycin-inducible macrolide resistance in S. aureus, which does not involve signal transduction.
Figures

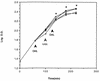
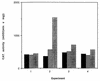
 ) min.
) min.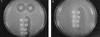
Similar articles
-
Glycerol monolaurate inhibits the production of beta-lactamase, toxic shock toxin-1, and other staphylococcal exoproteins by interfering with signal transduction.J Bacteriol. 1994 Jul;176(14):4204-9. doi: 10.1128/jb.176.14.4204-4209.1994. J Bacteriol. 1994. PMID: 8021206 Free PMC article.
-
Antibacterial synergy of glycerol monolaurate and aminoglycosides in Staphylococcus aureus biofilms.Antimicrob Agents Chemother. 2014 Nov;58(11):6970-3. doi: 10.1128/AAC.03672-14. Epub 2014 Sep 2. Antimicrob Agents Chemother. 2014. PMID: 25182634 Free PMC article.
-
Modulation of immune cell proliferation by glycerol monolaurate.Clin Diagn Lab Immunol. 1996 Jan;3(1):10-3. doi: 10.1128/cdli.3.1.10-13.1996. Clin Diagn Lab Immunol. 1996. PMID: 8770497 Free PMC article.
-
Vancomycin resistant Enterococci: A brief review.J Pak Med Assoc. 2018 May;68(5):768-772. J Pak Med Assoc. 2018. PMID: 29885179 Review.
-
Vancomycin resistance in gram-positive cocci.Clin Infect Dis. 2006 Jan 1;42 Suppl 1:S25-34. doi: 10.1086/491711. Clin Infect Dis. 2006. PMID: 16323116 Review.
Cited by
-
Short-chain fatty acids inhibit bacterial plasmid transfer through conjugation in vitro and in ex vivo chicken tissue explants.Front Microbiol. 2024 Jun 6;15:1414401. doi: 10.3389/fmicb.2024.1414401. eCollection 2024. Front Microbiol. 2024. PMID: 38903782 Free PMC article.
-
Surfactants, aromatic and isoprenoid compounds, and fatty acid biosynthesis inhibitors suppress Staphylococcus aureus production of toxic shock syndrome toxin 1.Antimicrob Agents Chemother. 2009 May;53(5):1898-906. doi: 10.1128/AAC.01293-08. Epub 2009 Feb 17. Antimicrob Agents Chemother. 2009. PMID: 19223628 Free PMC article.
-
Regulation of the expression of cell wall stress stimulon member gene msrA1 in methicillin-susceptible or -resistant Staphylococcus aureus.Antimicrob Agents Chemother. 2004 Aug;48(8):3057-63. doi: 10.1128/AAC.48.8.3057-3063.2004. Antimicrob Agents Chemother. 2004. PMID: 15273121 Free PMC article.
-
The effect of skin fatty acids on Staphylococcus aureus.Arch Microbiol. 2015 Mar;197(2):245-67. doi: 10.1007/s00203-014-1048-1. Epub 2014 Oct 18. Arch Microbiol. 2015. PMID: 25325933 Free PMC article.
-
Inhibitory activity of monoacylglycerols on biofilm formation in Aeromonas hydrophila, Streptococcus mutans, Xanthomonas oryzae, and Yersinia enterocolitica.Springerplus. 2016 Sep 9;5(1):1526. doi: 10.1186/s40064-016-3182-5. eCollection 2016. Springerplus. 2016. PMID: 27652099 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

