Differential responses of human mononuclear phagocytes to mycobacterial lipoarabinomannans: role of CD14 and the mannose receptor
- PMID: 9423835
- PMCID: PMC107854
- DOI: 10.1128/IAI.66.1.28-35.1998
Differential responses of human mononuclear phagocytes to mycobacterial lipoarabinomannans: role of CD14 and the mannose receptor
Abstract
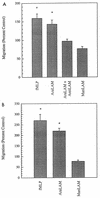
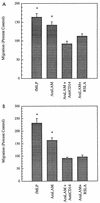
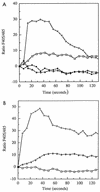
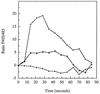
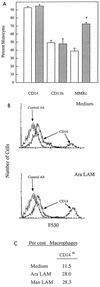
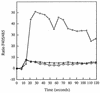
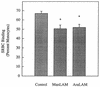
CD14 is a signaling receptor for both gram-negative bacterial lipopolysaccharide (LPS) and mycobacterial lipoarabinomannan (LAM) that lacks terminal mannosyl units (AraLAM). In contrast, terminally mannosylated LAM (ManLAM) binds the macrophage mannose receptor (MMRc), although the ability of the MMRc to serve as a signaling receptor has not been previously reported. We compared the abilities of AraLAM and ManLAM to induce distinct responses in two monocytic cell populations, freshly isolated human peripheral blood monocytes (PBM) and monocyte-derived macrophages (MDM). The responses examined were chemotaxis and transient changes in free cytosolic calcium ([Ca2+]in). We found that AraLAM but not ManLAM was chemotactic for both PBM and MDM. Migration of these cells in vitro to AraLAM was specifically blocked by an anti-CD14 monoclonal antibody, suggesting that CD14 mediates the chemotactic response to AraLAM. Subsequently, we found that AraLAM induced a transient rise in [Ca2+]in levels within a subpopulation of PBM but not MDM. This response was blocked by anti-CD14 antibodies. In contrast, ManLAM induced a transient rise in [Ca2+]in levels within a subpopulation of MDM but not PBM. This response was blocked by either anti-CD14 or anti-MMRc antibodies. These data suggest that the MMRc can serve as a signaling receptor and that coligation of both CD14 and the MMRc is required to elicit a specific response. Thus, one response to LAM (chemotaxis) can be elicited solely by engaging CD14, whereas a different response (changes in [Ca2+]in levels) depends on both the differentiation state of the cells and concomitant engagement of CD14 and the MMRc.
Figures
Similar articles
-
Mycobacterial lipoarabinomannan affects human polymorphonuclear and mononuclear phagocyte functions differently.Haematologica. 2000 Jan;85(1):11-8. Haematologica. 2000. PMID: 10629585
-
Binding of the terminal mannosyl units of lipoarabinomannan from a virulent strain of Mycobacterium tuberculosis to human macrophages.J Immunol. 1994 Apr 15;152(8):4070-9. J Immunol. 1994. PMID: 8144972
-
Differences in mannose receptor-mediated uptake of lipoarabinomannan from virulent and attenuated strains of Mycobacterium tuberculosis by human macrophages.J Immunol. 1996 Nov 15;157(10):4568-75. J Immunol. 1996. PMID: 8906835
-
Mannose-capped lipoarabinomannan in Mycobacterium tuberculosis pathogenesis.Pathog Dis. 2018 Jun 1;76(4):fty026. doi: 10.1093/femspd/fty026. Pathog Dis. 2018. PMID: 29722821 Free PMC article. Review.
-
Complex encounters at the macrophage-mycobacterium interface: studies on the role of the mannose receptor and CD14 in experimental infection models with Mycobacterium avium.Immunobiology. 2001 Dec;204(5):558-71. doi: 10.1078/0171-2985-00093. Immunobiology. 2001. PMID: 11846219 Review.
Cited by
-
Expression, secretion, and glycosylation of the 45- and 47-kDa glycoprotein of Mycobacterium tuberculosis in Streptomyces lividans.Appl Environ Microbiol. 2004 Feb;70(2):679-85. doi: 10.1128/AEM.70.2.679-685.2004. Appl Environ Microbiol. 2004. PMID: 14766542 Free PMC article.
-
Limited role of the Toll-like receptor-2 in resistance to Mycobacterium avium.Immunology. 2004 Feb;111(2):179-85. doi: 10.1111/j.0019-2805.2003.01807.x. Immunology. 2004. PMID: 15027903 Free PMC article.
-
CD14 directs adventitial macrophage precursor recruitment: role in early abdominal aortic aneurysm formation.J Am Heart Assoc. 2013 Mar 8;2(2):e000065. doi: 10.1161/JAHA.112.000065. J Am Heart Assoc. 2013. PMID: 23537804 Free PMC article.
-
The presence of CD14 overcomes evasion of innate immune responses by virulent Francisella tularensis in human dendritic cells in vitro and pulmonary cells in vivo.Infect Immun. 2010 Jan;78(1):154-67. doi: 10.1128/IAI.00750-09. Epub 2009 Oct 19. Infect Immun. 2010. PMID: 19841074 Free PMC article.
-
Macrophages in rheumatoid arthritis.Arthritis Res. 2000;2(3):189-202. doi: 10.1186/ar86. Epub 2000 Apr 12. Arthritis Res. 2000. PMID: 11094428 Free PMC article. Review.
References
-
- Barnes P F, Chatterjee D, Abrams J S, Lu S, Wang E, Yamamura M, Brennan P J, Modlin R L. Cytokine production induced by Mycobacterium tuberculosis lipoarabinomannan. Relationship to chemical structure. J Immunol. 1992;149:541–547. - PubMed
-
- Beekhuizen H, Blokl I, van Furth R. Cross-linking of CD14 molecules on monocytes results in a CD11/CD18- and ICAM-1-dependent adherence to cytokine-stimulated human endothelial cells. J Immunol. 1993;150:950–959. - PubMed
-
- Berman J S, Blumenthal R L, Kornfeld H, Cook J A, Cruikshank W W, Vermeulen M W, Chatterjee D, Belisle J T, Fenton M J. Chemotactic activity of mycobacterial lipoarabinomannans for human blood T lymphocytes in vitro. J Immunol. 1996;156:3828–3835. - PubMed
-
- Bernardo J, Brink H, Simons E R. Time dependence of transmembrane potential changes and intracellular calcium fluxes in stimulated human monocytes. J Cell Physiol. 1988;134:131–136. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

