Role of flagella in pathogenesis of Pseudomonas aeruginosa pulmonary infection
- PMID: 9423837
- PMCID: PMC107856
- DOI: 10.1128/IAI.66.1.43-51.1998
Role of flagella in pathogenesis of Pseudomonas aeruginosa pulmonary infection
Abstract
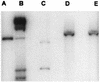
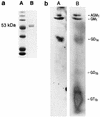
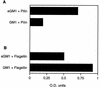
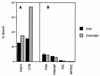
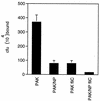
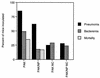
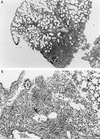
Pseudomonas aeruginosa strains are opportunistic pathogens associated with infections in immunocompromised hosts and patients with cystic fibrosis. Like many other mucosal pathogens, P. aeruginosa cells express flagella which provide motility and chemotaxis toward preferred substrates but also provide a ligand for clearance by phagocytic cells. We tested the role of flagella in the initial stages of respiratory tract infection by comparing the virulence of fliC mutants in a neonatal mouse model of pneumonia. In the absence of fliC, there was no mortality, compared with 30% mortality attributed to the parental strain PAK or 15% mortality associated with infection due to a pilA mutant PAK/NP (P < 0.0001). The fliC mutants caused pneumonia in only 25% of the mice inoculated, regardless of whether there was expression of the pilus, whereas the parental strain was associated with an 80% rate of pneumonia. Histopathological studies demonstrated that the fliC mutants caused very focal inflammation and that the organisms did not spread through the lungs as seen in infection due to either PAK or PAK/NP. Purified flagellin elicited an intense inflammatory response in the mouse lung. 125I-labeled flagellin bound to the glycolipids GM1 and GD1a and to asialoGM1 in an in vitro binding assay. However, flagellin-mediated binding to epithelial gangliosides was a relatively unusual event, as quantified by binding assays of wild-type or fliC mutant organisms to CHO Lec-2 cells with membrane-incorporated GM1. Fla+ organisms but not fliC mutants were efficiently taken up by murine macrophages. P. aeruginosa flagella are important in the establishment of respiratory tract infection and may act as a tether in initial interactions with epithelial membranes. This function is offset by the contribution of flagella to host clearance mechanisms facilitating phagocytic clearance and the role of flagellar genes in mucin binding and clearance.
Figures



References
-
- Akerley B J, Cotter P A, Miller J F. Ectopic expression of the flagellar regulon alters development of the Bordetella-host interaction. Cell. 1995;80:611–620. - PubMed
-
- Bargouthi S, Everett K D E, Speert D P. Nonopsonic phagocytosis of Pseudomonas aeruginosa requires facilitated transport of d-glucose by macrophages. J Immunol. 1995;154:3420–3428. - PubMed
-
- Brimer CD, Kelly-Wintenberg K, Montie T C. Abstracts of the 97th General Meeting of the American Society for Microbiology 1997. Washington, D.C: American Society for Microbiology; 1997. Cloning and characterization of P. aeruginosa a-type flagellin genes, abstr. D-43.
-
- Cormack B P, Valdivia R H, Falkow S. FACS-optimized mutants of the green fluorescent protein (GFP) Gene. 1996;173:33–38. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

