Binding of salivary glycoprotein-secretory immunoglobulin A complex to the surface protein antigen of Streptococcus mutans
- PMID: 9423847
- PMCID: PMC107866
- DOI: 10.1128/IAI.66.1.115-121.1998
Binding of salivary glycoprotein-secretory immunoglobulin A complex to the surface protein antigen of Streptococcus mutans
Abstract
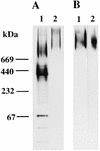
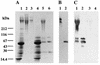
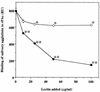
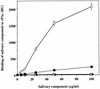
The interaction between a surface protein antigen (PAc) of Streptococcus mutans and human salivary agglutinin was analyzed with a surface plasmon resonance biosensor. The major component sugars of the salivary agglutinin were galactose, fucose, mannose, N-acetylglucosamine, N-acetylgalactosamine, and N-acetylneuraminic acid. Binding of salivary agglutinin to PAc was calcium dependent and heat labile and required a pH greater than 5. Binding was significantly inhibited by N-acetylneuraminic acid and alpha2,6-linked sialic acid-specific lectin derived from Sambucus sieboldiana in a dose-dependent manner. Pretreatment of the salivary agglutinin with sialidase reduced the binding activity of the agglutinin to the PAc molecule. The agglutinin was dissociated into high-molecular-mass glycoprotein and secretory immunoglobulin A (sIgA) components by electrophoretic fractionation in the presence of 1% sodium dodecyl sulfate and 1% 2-mercaptoethanol. Neither of the components separated by electrophoretic fractionation, high-molecular-mass glycoprotein or sIgA, bound to the PAc molecule. Furthermore, the high-molecular-mass glycoprotein strongly inhibited the binding of the native salivary complex to PAc. These results suggest that the complex formed by the high-molecular-mass salivary glycoprotein and sIgA is essential for the binding reaction and that the sialic acid residues of the complex play an important role in the interaction between the agglutinin and PAc of S. mutans.
Figures


Similar articles
-
Differentiation and interaction of secretory immunoglobulin A and a calcium-dependent parotid agglutinin for several bacterial strains.Infect Immun. 1987 Feb;55(2):288-92. doi: 10.1128/iai.55.2.288-292.1987. Infect Immun. 1987. PMID: 3100447 Free PMC article.
-
Characterization of a rat salivary sialoglycoprotein complex which agglutinates Streptococcus mutans.Infect Immun. 1987 May;55(5):1264-73. doi: 10.1128/iai.55.5.1264-1273.1987. Infect Immun. 1987. PMID: 3570462 Free PMC article.
-
Characterization of a salivary agglutinin reacting with a serotype c strain of Streptococcus mutans.Eur J Biochem. 1983 Jun 15;133(2):255-61. doi: 10.1111/j.1432-1033.1983.tb07456.x. Eur J Biochem. 1983. PMID: 6852037
-
Saliva-binding region of Streptococcus mutans surface protein antigen.Infect Immun. 1993 Oct;61(10):4344-9. doi: 10.1128/iai.61.10.4344-4349.1993. Infect Immun. 1993. PMID: 8406823 Free PMC article.
-
Isolation and characterization of a 60-kilodalton salivary glycoprotein with agglutinating activity against strains of Streptococcus mutans.Infect Immun. 1986 Feb;51(2):405-13. doi: 10.1128/iai.51.2.405-413.1986. Infect Immun. 1986. PMID: 3002983 Free PMC article.
Cited by
-
The scavenger receptor, cysteine-rich domain-containing molecule gp-340 is differentially regulated in epithelial cell lines by phorbol ester.Clin Exp Immunol. 2002 Dec;130(3):449-58. doi: 10.1046/j.1365-2249.2002.01992.x. Clin Exp Immunol. 2002. PMID: 12452835 Free PMC article.
-
A peptide domain of bovine milk lactoferrin inhibits the interaction between streptococcal surface protein antigen and a salivary agglutinin peptide domain.Infect Immun. 2004 Oct;72(10):6181-4. doi: 10.1128/IAI.72.10.6181-6184.2004. Infect Immun. 2004. PMID: 15385529 Free PMC article.
-
Immunogenicity and in vitro and in vivo protective effects of antibodies targeting a recombinant form of the Streptococcus mutans P1 surface protein.Infect Immun. 2014 Dec;82(12):4978-88. doi: 10.1128/IAI.02074-14. Epub 2014 Sep 15. Infect Immun. 2014. PMID: 25225243 Free PMC article.
-
Binding of the streptococcal surface glycoproteins GspB and Hsa to human salivary proteins.Infect Immun. 2006 Mar;74(3):1933-40. doi: 10.1128/IAI.74.3.1933-1940.2006. Infect Immun. 2006. PMID: 16495569 Free PMC article.
-
Expression and functional properties of the Streptococcus intermedius surface protein antigen I/II.Infect Immun. 2001 Jul;69(7):4647-53. doi: 10.1128/IAI.69.7.4647-4653.2001. Infect Immun. 2001. PMID: 11402009 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

