Identification and characterization of protective T cells in hsp65 DNA-vaccinated and Mycobacterium tuberculosis-infected mice
- PMID: 9423854
- PMCID: PMC107873
- DOI: 10.1128/IAI.66.1.169-175.1998
Identification and characterization of protective T cells in hsp65 DNA-vaccinated and Mycobacterium tuberculosis-infected mice
Abstract
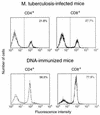
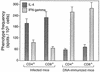
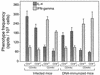
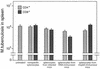
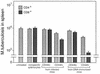
Immunization by intramuscular injection of plasmid DNA expressing mycobacterial 65-kDa heat shock protein (hsp65) protects mice against challenge with virulent Mycobacterium tuberculosis H37Rv. During infection or after immunization, CD4+/CD8- and CD8+/CD4- hsp65-reactive T cells increased equally in spleens. During infection, the majority of these cells were weakly CD44 positive (CD44(lo)) and produced interleukin 4 (IL-4) whereas after immunization the majority were highly CD44 positive (CD44(hi)) and produced gamma interferon (IFN-gamma). In adoptive transfer of protection to naive mice, the total CD8+/CD4- cell population purified from spleens of immunized mice was more protective than that from infected mice. When the cells were separated into CD4+/CD8- and CD8+/CD4- types and then into CD44(hi) and CD44(lo) types, CD44(lo) cells were essentially unable to transfer protection, the most protective CD44(hi) cells were CD8+/CD4-, and those from immunized mice were much more protective than those from infected mice. Thus, whereas the CD44(lo) IL-4-producing phenotype prevailed during infection, protection was associated with the CD8+/CD44(hi) IFN-gamma-producing phenotype that predominated after immunization. This conclusion was confirmed and extended by analysis of 16 hsp65-reactive T-cell clones from infected mice and 16 from immunized mice; the most protective clones, in addition, displayed antigen-specific cytotoxicity.
Figures


References
-
- Cron R Q, Gajewski T F, Sharrow S O, Fitch F W, Matis L A, Bluestone J A. Phenotypic and functional analysis of murine CD3+, CD4−, CD8−, TCR-gamma/delta-expressing peripheral T cells. J Immunol. 1989;142:3754–3762. - PubMed
-
- Dutton R W. The regulation of the development of CD8 effector T cells. J Immunol. 1996;157:4287–4292. - PubMed
-
- Ernst D N, Weigle W O, Noonan D J, McQuitty D N, Hobbs M V. The age-associated increase in IFN-gamma synthesis by mouse CD8+ T cells correlates with shifts in the frequencies of cell subsets defined by membrane CD44, CD45RB, 3G11, and MEL-14 expression. J Immunol. 1993;151:575–587. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

