Activated pulmonary macrophages are insufficient for resistance against Pneumocystis carinii
- PMID: 9423872
- PMCID: PMC107891
- DOI: 10.1128/IAI.66.1.305-314.1998
Activated pulmonary macrophages are insufficient for resistance against Pneumocystis carinii
Abstract
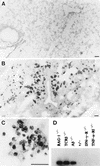
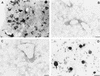
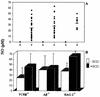
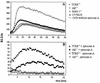
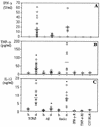
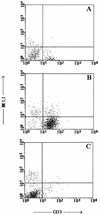
CD4+ T cells are pivotal for elimination of Pneumocystis carinii from infected lungs, and alveolar macrophages are considered the main effector cells clearing the infected host of P. carinii organisms. To investigate this issue, several mutant mouse strains were used in a previously established experimental setup which facilitates natural acquisition of disease through inhalation of airborne fungal organisms. Mutant mice deficient in major histocompatibility complex class II molecules (A beta(-/-)), T-cell receptor alphabeta cells (TCR beta(-/-)), or all mature T and B lymphocytes (RAG-1(-/-)) were naturally susceptible to P. carinii, whereas mouse mutants lacking the gamma interferon (IFN-gamma) receptor (IFN-gamma-R(-/-)) or tumor necrosis factor alpha (TNF-alpha) type I receptor (p55) (TNF-alpha-RI(-/-)) resisted disease acquisition. Analysis of pulmonary cytokine patterns and free radical expression revealed the presence of superoxide, nitric oxide, and interleukin-1 (IL-1) mRNA and elevated levels of IFN-gamma, TNF-alpha, and IL-12 in diseased TCR beta(-/-) and RAG-1(-/-) mice. Pulmonary macrophages of all diseased mouse mutants expressed scavenger and mannose receptors. Morbid A beta(-/-) mutants displayed significant NO levels and IL-1 mRNA only, whereas heterozygous controls did not exhibit any signs of disease. Interestingly, neither IFN-gamma nor TNF-alpha appeared to be essential for resisting natural infection with P. carinii, nor were these cytokines sufficient for mediating resistance during established disease in the absence of CD4+ T lymphocytes. Taken together, the results indicated that an activated phagocyte system, as evidenced by cytokine and NO secretion, in diseased mutants was apparently operative but did not suffice for parasite clearance in the absence of CD4+ TCR alphabeta cells. Therefore, additional pathways, possibly involving interactions of inflammatory cytokines with CD4+ T lymphocytes, must contribute to successful resistance against P. carinii.
Figures


Similar articles
-
Neither classical nor alternative macrophage activation is required for Pneumocystis clearance during immune reconstitution inflammatory syndrome.Infect Immun. 2015 Dec;83(12):4594-603. doi: 10.1128/IAI.00763-15. Epub 2015 Sep 14. Infect Immun. 2015. PMID: 26371121 Free PMC article.
-
Naturally acquired Pneumocystis carinii pneumonia in gene disruption mutant mice: roles of distinct T-cell populations in infection.Infect Immun. 1996 Aug;64(8):3201-9. doi: 10.1128/iai.64.8.3201-3209.1996. Infect Immun. 1996. PMID: 8757854 Free PMC article.
-
Effect on parasite eradication of Pneumocystis carinii-specific antibodies produced in the presence or absence of CD4(+) alphabeta T lymphocytes.Eur J Immunol. 1999 Aug;29(8):2464-75. doi: 10.1002/(SICI)1521-4141(199908)29:08<2464::AID-IMMU2464>3.0.CO;2-F. Eur J Immunol. 1999. PMID: 10458760
-
Mechanisms of defence in the lung: lessons from Pneumocystis carinii pneumonia.Sarcoidosis Vasc Diffuse Lung Dis. 2000 Jun;17(2):130-9. Sarcoidosis Vasc Diffuse Lung Dis. 2000. PMID: 10957761 Review.
-
Cell-mediated adaptive immune defense of the lungs.Proc Am Thorac Soc. 2005;2(5):412-6. doi: 10.1513/pats.200507-070JS. Proc Am Thorac Soc. 2005. PMID: 16322591 Free PMC article. Review.
Cited by
-
Interleukin-23 (IL-23)-IL-17 cytokine axis in murine Pneumocystis carinii infection.Infect Immun. 2007 Jun;75(6):3055-61. doi: 10.1128/IAI.01329-06. Epub 2007 Apr 2. Infect Immun. 2007. PMID: 17403873 Free PMC article.
-
Resistance of virulent Mycobacterium avium to gamma interferon-mediated antimicrobial activity suggests additional signals for induction of mycobacteriostasis.Infect Immun. 1999 Jul;67(7):3610-8. doi: 10.1128/IAI.67.7.3610-3618.1999. Infect Immun. 1999. PMID: 10377146 Free PMC article.
-
Down-regulation of GATA-2 transcription during Pneumocystis carinii infection.Infect Immun. 2000 Aug;68(8):4720-4. doi: 10.1128/IAI.68.8.4720-4724.2000. Infect Immun. 2000. PMID: 10899878 Free PMC article.
-
Immunological features of Pneumocystis carinii infection in humans.Clin Diagn Lab Immunol. 1999 Mar;6(2):149-55. doi: 10.1128/CDLI.6.2.149-155.1999. Clin Diagn Lab Immunol. 1999. PMID: 10066645 Free PMC article. Review. No abstract available.
-
Intrinsic Programming of Alveolar Macrophages for Protective Antifungal Innate Immunity Against Pneumocystis Infection.Front Immunol. 2018 Sep 19;9:2131. doi: 10.3389/fimmu.2018.02131. eCollection 2018. Front Immunol. 2018. PMID: 30283457 Free PMC article.
References
-
- Abe E, Ishimi Y, Tanaka H, Miyaura C, Nagasawa H, Hayashi T, Suda T. The relationship between fusion and proliferation in mouse alveolar macrophages. Endocrinology. 1986;121:271–277. - PubMed
-
- Bendelac A, Rivera M N, Park S-H, Roark J H. Mouse CD1-specific NK cells: development, specificity, function. Annu Rev Immunol. 1997;15:535–562. - PubMed
-
- Celada A, Nathan C. Macrophage activation revisited. Immunol Today. 1994;15:100–102. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

