Use of green fluorescent protein to assess urease gene expression by uropathogenic Proteus mirabilis during experimental ascending urinary tract infection
- PMID: 9423875
- PMCID: PMC107894
- DOI: 10.1128/IAI.66.1.330-335.1998
Use of green fluorescent protein to assess urease gene expression by uropathogenic Proteus mirabilis during experimental ascending urinary tract infection
Abstract
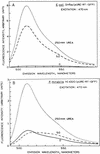
Proteus mirabilis, a cause of complicated urinary tract infection, expresses urease when exposed to urea. While it is recognized that the positive transcriptional activator UreR induces gene expression, the levels of expression of the enzyme during experimental infection are not known. To investigate in vivo expression of P. mirabilis urease, the gene encoding green fluorescent protein (GFP) was used to construct reporter fusions. Translational fusions of urease accessory gene ureD, which is preceded by a urea-inducible promoter, were made with gfp (modified to express S65T/V68L/S72A [B. P. Cormack et al. Gene 173:33-38, 1996]). Constructs were confirmed by sequencing of the fusion junctions. UreD-GFP fusion protein was induced by urea in both Escherichia coli DH5alpha and P. mirabilis HI4320. By using Western blotting with antiserum raised against GFP, expression level was shown to correlate with urea concentration (tested from 0 to 500 mM), with highest induction at 200 to 500 mM urea. Fluorescent E. coli and P. mirabilis bacteria were observed by fluorescence microscopy following urea induction, and the fluorescence intensity of GFP in cell lysates was measured by spectrophotofluorimetry. P. mirabilis HI4320 carrying the UreD-GFP fusion plasmid was transurethrally inoculated into the bladders of CBA mice. One week postchallenge, fluorescent bacteria were detected in thin sections of both bladder and kidney samples; the fluorescence intensity of bacteria in bladder tissue was higher than that in the kidney. Kidneys were primarily infected with single-cell-form fluorescent bacteria, while aggregated bacterial clusters were observed in the bladder. Elongated swarmer cells were only rarely observed. These observations demonstrate that urease is expressed in vivo and that using GFP as a reporter protein is a viable approach to investigate in vivo expression of P. mirabilis virulence genes in experimental urinary tract infection.
Figures


Similar articles
-
From Catheter to Kidney Stone: The Uropathogenic Lifestyle of Proteus mirabilis.Trends Microbiol. 2017 Apr;25(4):304-315. doi: 10.1016/j.tim.2016.11.015. Epub 2016 Dec 22. Trends Microbiol. 2017. PMID: 28017513 Free PMC article. Review.
-
In vivo phase variation of MR/P fimbrial gene expression in Proteus mirabilis infecting the urinary tract.Mol Microbiol. 1997 Mar;23(5):1009-19. doi: 10.1046/j.1365-2958.1997.2791645.x. Mol Microbiol. 1997. PMID: 9076737
-
UreR, the transcriptional activator of the Proteus mirabilis urease gene cluster, is required for urease activity and virulence in experimental urinary tract infections.Infect Immun. 2003 Feb;71(2):1026-30. doi: 10.1128/IAI.71.2.1026-1030.2003. Infect Immun. 2003. PMID: 12540589 Free PMC article.
-
Differential regulation of the Proteus mirabilis urease gene cluster by UreR and H-NS.Microbiology (Reading). 2003 Dec;149(Pt 12):3383-3394. doi: 10.1099/mic.0.26624-0. Microbiology (Reading). 2003. PMID: 14663072
-
Virulence determinants of uropathogenic Escherichia coli and Proteus mirabilis.Kidney Int Suppl. 1994 Nov;47:S129-36. Kidney Int Suppl. 1994. PMID: 7869662 Review.
Cited by
-
Measurement of bacterial gene expression in vivo.Philos Trans R Soc Lond B Biol Sci. 2000 May 29;355(1397):601-11. doi: 10.1098/rstb.2000.0601. Philos Trans R Soc Lond B Biol Sci. 2000. PMID: 10874733 Free PMC article. Review.
-
From Catheter to Kidney Stone: The Uropathogenic Lifestyle of Proteus mirabilis.Trends Microbiol. 2017 Apr;25(4):304-315. doi: 10.1016/j.tim.2016.11.015. Epub 2016 Dec 22. Trends Microbiol. 2017. PMID: 28017513 Free PMC article. Review.
-
Predictive and interpretive simulation of green fluorescent protein expression in reporter bacteria.J Bacteriol. 2001 Dec;183(23):6752-62. doi: 10.1128/JB.183.23.6752-6762.2001. J Bacteriol. 2001. PMID: 11698362 Free PMC article.
-
Proteus mirabilis and Urinary Tract Infections.Microbiol Spectr. 2015 Oct;3(5):10.1128/microbiolspec.UTI-0017-2013. doi: 10.1128/microbiolspec.UTI-0017-2013. Microbiol Spectr. 2015. PMID: 26542036 Free PMC article. Review.
-
Proteus mirabilis UreR coordinates cellular functions required for urease activity.J Bacteriol. 2024 Apr 18;206(4):e0003124. doi: 10.1128/jb.00031-24. Epub 2024 Mar 27. J Bacteriol. 2024. PMID: 38534115 Free PMC article.
References
-
- Allison C, Emody L, Coleman N, Hughes C. The role of swarm cell differentiation and multicellular migration in the uropathogenicity of Proteus mirabilis. J Infect Dis. 1994;169:1155–1158. - PubMed
-
- Allison C, Lai H-C, Hughes C. Co-ordinate expression of virulence genes during swarm cell differentiation and population migration of Proteus mirabilis. Mol Microbiol. 1992;6:1583–1591. - PubMed
-
- Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K, editors. Current protocols in molecular biology. New York, N.Y: John Wiley & Sons, Inc.; 1995.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

