Identification of amino acid residues of the NR2A subunit that control glutamate potency in recombinant NR1/NR2A NMDA receptors
- PMID: 9425000
- PMCID: PMC6792534
- DOI: 10.1523/JNEUROSCI.18-02-00581.1998
Identification of amino acid residues of the NR2A subunit that control glutamate potency in recombinant NR1/NR2A NMDA receptors
Abstract
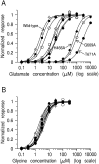
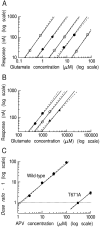
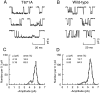
The NMDA type of ligand-gated glutamate receptor requires the presence of both glutamate and glycine for gating. These receptors are hetero-oligomers of NR1 and NR2 subunits. Previously it was thought that the binding sites for glycine and glutamate were formed by residues on the NR1 subunit. Indeed, it has been shown that the effects of glycine are controlled by residues on the NR1 subunit, and a "Venus flytrap" model for the glycine binding site has been suggested by analogy with bacterial periplasmic amino acid binding proteins. By analysis of 10 mutant NMDA receptors, we now show that residues on the NR2A subunit control glutamate potency in recombinant NR1/NR2A receptors, without affecting glycine potency. Furthermore, we provide evidence that, at least for some mutated residues, the reduced potency of glutamate cannot be explained by alteration of gating but has to be caused primarily by impairing the binding of the agonist to the resting state of the receptor. One NR2A mutant, NR2A(T671A), had an EC50 for glutamate 1000-fold greater than wild type and a 255-fold reduced affinity for APV, yet it had single-channel openings very similar to those of wild type. Therefore we propose that the glutamate binding site is located on NR2 subunits and (taking our data together with previous work) is not on the NR1 subunit. Our data further imply that each NMDA receptor subunit possesses a binding site for an agonist (glutamate or glycine).
Figures



References
-
- Béhé P, Stern P, Wyllie DJ, Nassar M, Schoepfer R, Colquhoun D. Determination of NMDA NR1 subunit copy number in recombinant NMDA receptors. Proc R Soc Lond [Biol] 1995;262:205–213. - PubMed
-
- Choi DW. Glutamate neurotoxicity and diseases of the nervous system. Neuron. 1988;1:623–634. - PubMed
-
- Collingridge GL, Bliss TV. Memories of NMDA receptors and LTP. Trends Neurosci. 1995;18:54–56. - PubMed
-
- Colquhoun D, Farrant M. The binding issue. Nature. 1993;366:510–511. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
