Intracellular calcium regulates agrin-induced acetylcholine receptor clustering
- PMID: 9425009
- PMCID: PMC6792545
- DOI: 10.1523/JNEUROSCI.18-02-00672.1998
Intracellular calcium regulates agrin-induced acetylcholine receptor clustering
Abstract
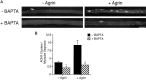
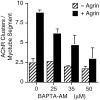
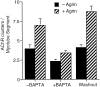
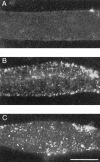
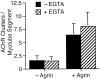
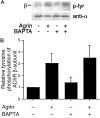
Agrin is an extracellular matrix protein that directs neuromuscular junction formation. Early signal transduction events in agrin-mediated postsynaptic differentiation include activation of a receptor tyrosine kinase and phosphorylation of acetylcholine receptors (AChRs), but later steps in this pathway are unknown. Here, we have investigated the role of intracellular calcium in agrin-induced AChR clustering on cultured myotubes. Clamping intracellular calcium levels by loading with the fast chelator BAPTA inhibited agrin-induced AChR aggregation. In addition, preexisting AChR aggregates dispersed under these conditions, indicating that the maintenance of AChR clusters is similarly dependent on intracellular calcium fluxes. The decrease in AChR clusters in BAPTA-loaded cells was dose-dependent and reversible, and no change in the number or mobility of AChRs was observed. Clamping intracellular calcium did not block agrin-induced tyrosine phosphorylation of the AChR beta-subunit, indicating that intracellular calcium fluxes are likely to act downstream from or parallel to AChR phosphorylation. Finally, the targets of the intracellular calcium are likely to be close to the calcium source, since agrin-induced AChR clustering was unaffected in cells loaded with EGTA, a slower-binding calcium chelator. These findings distinguish a novel step in the signal transduction mechanism of agrin and raise the possibility that the pathways mediating agrin- and activity-driven changes in synaptic architecture could intersect at the level of intracellular calcium fluxes.
Figures
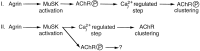
Similar articles
-
Calcium-dependent maintenance of agrin-induced postsynaptic specializations.Neuroscience. 2003;122(3):659-68. doi: 10.1016/s0306-4522(03)00602-x. Neuroscience. 2003. PMID: 14622909
-
Tyrosine phosphatases such as SHP-2 act in a balance with Src-family kinases in stabilization of postsynaptic clusters of acetylcholine receptors.BMC Neurosci. 2007 Jul 2;8:46. doi: 10.1186/1471-2202-8-46. BMC Neurosci. 2007. PMID: 17605785 Free PMC article.
-
Sialic acid inhibits agrin signaling in C2 myotubes.Cell Tissue Res. 2000 Feb;299(2):273-9. doi: 10.1007/s004419900146. Cell Tissue Res. 2000. PMID: 10741468
-
Development of the neuromuscular junction.Cell Tissue Res. 2006 Nov;326(2):263-71. doi: 10.1007/s00441-006-0237-x. Epub 2006 Jul 4. Cell Tissue Res. 2006. PMID: 16819627 Review.
-
Regulation of ligand-gated ion channels by protein phosphorylation.Adv Second Messenger Phosphoprotein Res. 1999;33:49-78. doi: 10.1016/s1040-7952(99)80005-6. Adv Second Messenger Phosphoprotein Res. 1999. PMID: 10218114 Review.
Cited by
-
MuSK signaling at the neuromuscular junction.J Mol Neurosci. 2006;30(1-2):223-6. doi: 10.1385/JMN:30:1:223. J Mol Neurosci. 2006. PMID: 17192681
-
Muscle-wide secretion of a miniaturized form of neural agrin rescues focal neuromuscular innervation in agrin mutant mice.Proc Natl Acad Sci U S A. 2008 Aug 12;105(32):11406-11. doi: 10.1073/pnas.0801683105. Epub 2008 Aug 6. Proc Natl Acad Sci U S A. 2008. PMID: 18685098 Free PMC article.
-
Src, Fyn, and Yes are not required for neuromuscular synapse formation but are necessary for stabilization of agrin-induced clusters of acetylcholine receptors.J Neurosci. 2001 May 1;21(9):3151-60. doi: 10.1523/JNEUROSCI.21-09-03151.2001. J Neurosci. 2001. PMID: 11312300 Free PMC article.
-
Agrin promotes synaptic differentiation by counteracting an inhibitory effect of neurotransmitter.Proc Natl Acad Sci U S A. 2005 Aug 2;102(31):11088-93. doi: 10.1073/pnas.0504806102. Epub 2005 Jul 25. Proc Natl Acad Sci U S A. 2005. PMID: 16043708 Free PMC article.
-
Evidence of an agrin receptor in cortical neurons.J Neurosci. 1999 Sep 1;19(17):7384-93. doi: 10.1523/JNEUROSCI.19-17-07384.1999. J Neurosci. 1999. PMID: 10460245 Free PMC article.
References
-
- Apel ED, Glass DJ, Moscoso LM, Yancopolous GD, Sanes JR. Rapsyn is required for MuSK signaling and recruits synaptic components to a MuSK-containing scaffold. Neuron. 1997;18:623–635. - PubMed
-
- Augustine GJ, Adler EM, Charlton MP, Hans M, Swandulla D, Zipser K. Presynaptic calcium signals during neurotransmitter release: detection with fluorescent indicators and other calcium chelators. J Physiol (Paris) 1992;86:129–134. - PubMed
-
- Bailey CH, Kandel ER. Structural changes accompanying memory storage. Annu Rev Physiol. 1993;55:397–426. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
