Overloaded endoplasmic reticulum-Golgi compartments, a possible pathomechanism of peripheral neuropathies caused by mutations of the peripheral myelin protein PMP22
- PMID: 9425015
- PMCID: PMC6792531
- DOI: 10.1523/JNEUROSCI.18-02-00731.1998
Overloaded endoplasmic reticulum-Golgi compartments, a possible pathomechanism of peripheral neuropathies caused by mutations of the peripheral myelin protein PMP22
Abstract
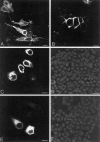
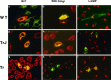
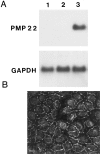
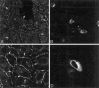
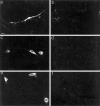
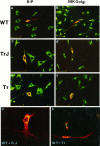
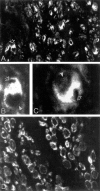
Nonconservative point mutations of the peripheral myelin protein 22 (PMP22) are associated with Charcot-Marie-Tooth type 1A disease, the most common inherited peripheral neuropathy in humans, and with the Trembler J (TrJ) and Trembler (Tr) alleles in mice. We investigated the intracellular transport of wild-type PMP22 and its TrJ and Tr mutant forms in Schwann cells and in a non-neuronal cell line. In contrast to wild type, mutant proteins were not inserted into the plasma membrane and accumulated in the endoplasmic reticulum and Golgi compartments. Coexpression of each mutant with wild-type PMP22 confirmed the different intracellular distribution of the mutant forms, indicating that neither the TrJ nor Tr protein has a dominant-negative effect on the cellular distribution of wild-type PMP22. Accumulation of PMP22 immunoreactivity in the cell body of myelinating Schwann cells was also observed in nerve biopsies obtained from CMT1A patients carrying the TrJ point mutation. We propose that impaired trafficking of mutated PMP22 affects Schwann cell physiology leading to myelin instability and loss.
Figures
References
-
- Adlkofer K, Martini R, Aguzzi A, Zielasek J, Toyka KV, Suter U. Hypermyelination and demyelinating peripheral neuropathy in PMP22-deficient mice. Nat Genet. 1995;11:274–280. - PubMed
-
- Baechner D, Liehr T, Hameister H, Altenberger H, Grehel H, Suter U, Rautenstrauss B. Widespread expression of the peripheral myelin protein-22 gene (PMP22) in neural and non-neural tissues during murine development. J Neurosci Res. 1995;42:733–741. - PubMed
-
- Cheng SH, Gregory RJ, Marshall J, Paul S, Souza DW, White GA, O’Riordan CR, Smith AE. Defective intracellular transport and processing of CFTR is the molecular basis of most cystic fibrosis. Cell. 1990;63:827–834. - PubMed
-
- Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–159. - PubMed
-
- De Jonghe P, Timmerman V, Nelis E, Martin J-J, Van Broeckhoven C. Charcot-Marie-Tooth disease and related peripheral neuropathies. J Periph Nerve System. 1997;2:370–387. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
