Fetal spinal cord transplants support growth of supraspinal and segmental projections after cervical spinal cord hemisection in the neonatal rat
- PMID: 9425019
- PMCID: PMC6792540
- DOI: 10.1523/JNEUROSCI.18-02-00779.1998
Fetal spinal cord transplants support growth of supraspinal and segmental projections after cervical spinal cord hemisection in the neonatal rat
Abstract
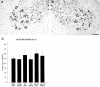
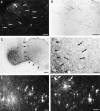
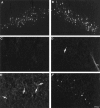
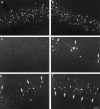
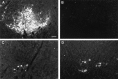
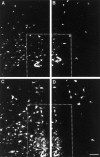
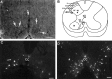
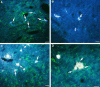
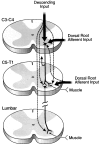
Cervical spinal cord injury at birth permanently disrupts forelimb function in goal-directed reaching. Transplants of fetal spinal cord tissue permit the development of skilled forelimb use and associated postural adjustments (, companion article). The aim of this study was to determine whether transplants of fetal spinal cord tissue support the remodeling of supraspinal and segmental pathways that may underlie recovery of postural reflexes and forelimb movements. Although brainstem-spinal and segmental projections to the cervical spinal cord are present at birth, skilled forelimb reaching has not yet developed. Three-day-old rats received a cervical spinal cord overhemisection with or without transplantation of fetal spinal cord tissue (embryonic day 14); unoperated pups served as normal controls. Neuroanatomical tracing techniques were used to examine the organization of CNS pathways that may influence target-directed reaching. In animals with hemisections only, corticospinal, brainstem-spinal, and dorsal root projections within the spinal cord were decreased in number and extent. In contrast, animals receiving hemisections plus transplants exhibited growth of these projections throughout the transplant and over long distances within the host spinal cord caudal to the transplant. Raphespinal axons were apposed to numerous propriospinal neurons in control and transplant animals; these associations were greatly reduced in the lesion-only animals. These observations suggest that after neonatal cervical spinal cord injury, embryonic transplants support axonal growth of CNS pathways and specifically supraspinal input to propriospinal neurons. We suggest that after neonatal spinal injury in the rat, the transplant-mediated reestablishment of supraspinal input to spinal circuitry is the mechanism underlying the development of target-directed reaching and associated postural adjustments.
Figures


Similar articles
-
Fetal spinal cord transplants support the development of target reaching and coordinated postural adjustments after neonatal cervical spinal cord injury.J Neurosci. 1998 Jan 15;18(2):763-78. doi: 10.1523/JNEUROSCI.18-02-00763.1998. J Neurosci. 1998. PMID: 9425018 Free PMC article.
-
Transplants and neurotrophic factors increase regeneration and recovery of function after spinal cord injury.Prog Brain Res. 2002;137:257-73. doi: 10.1016/s0079-6123(02)37020-1. Prog Brain Res. 2002. PMID: 12440372 Review.
-
Regenerating and sprouting axons differ in their requirements for growth after injury.Exp Neurol. 1997 Nov;148(1):51-72. doi: 10.1006/exnr.1997.6632. Exp Neurol. 1997. PMID: 9398450
-
Delayed intervention with transplants and neurotrophic factors supports recovery of forelimb function after cervical spinal cord injury in adult rats.J Neurotrauma. 2006 May;23(5):617-34. doi: 10.1089/neu.2006.23.617. J Neurotrauma. 2006. PMID: 16689666
-
Fetal cell grafts into resection and contusion/compression injuries of the rat and cat spinal cord.Exp Neurol. 1992 Jan;115(1):177-88. doi: 10.1016/0014-4886(92)90245-l. Exp Neurol. 1992. PMID: 1370221 Review.
Cited by
-
Zafirlukast in combination with pseudohypericin attenuates spinal cord injury and motor function in experimental mice.Drug Des Devel Ther. 2018 Aug 1;12:2389-2402. doi: 10.2147/DDDT.S154814. eCollection 2018. Drug Des Devel Ther. 2018. PMID: 30122897 Free PMC article.
-
Directing dopaminergic fiber growth along a preformed molecular pathway from embryonic ventral mesencephalon transplants in the rat brain.J Neurosci Res. 2011 May;89(5):619-27. doi: 10.1002/jnr.22575. Epub 2011 Feb 17. J Neurosci Res. 2011. PMID: 21337366 Free PMC article.
-
Fetal spinal cord transplants support the development of target reaching and coordinated postural adjustments after neonatal cervical spinal cord injury.J Neurosci. 1998 Jan 15;18(2):763-78. doi: 10.1523/JNEUROSCI.18-02-00763.1998. J Neurosci. 1998. PMID: 9425018 Free PMC article.
-
Degradation of chondroitin sulfate proteoglycans potentiates transplant-mediated axonal remodeling and functional recovery after spinal cord injury in adult rats.J Comp Neurol. 2006 Jul 10;497(2):182-98. doi: 10.1002/cne.20980. J Comp Neurol. 2006. PMID: 16705682 Free PMC article.
-
Direct agonists for serotonin receptors enhance locomotor function in rats that received neural transplants after neonatal spinal transection.J Neurosci. 1999 Jul 15;19(14):6213-24. doi: 10.1523/JNEUROSCI.19-14-06213.1999. J Neurosci. 1999. PMID: 10407057 Free PMC article.
References
-
- Alstermark B, Sasaki S. Integration in descending motor pathways controlling the forelimb in the cat. 13. Corticospinal effects in shoulder: elbow, wrist, and digit motoneurones. Exp Brain Res. 1985;59:353–364. - PubMed
-
- Alstermark B, Wessberg J. Timing of postural adjustment in relation to forelimb target-reaching in cats. Acta Physiol Scand. 1985;125:337–340. - PubMed
-
- Alstermark B, Lindstrom S, Lundberg A, Sybirska E. Integration in descending motor pathways controlling the forelimb in the cat. 8. Ascending projection to the lateral reticular nucleus from C3–C4 propriospinal neurones also projecting to forelimb motoneurones. Exp Brain Res. 1981a;42:282–298. - PubMed
-
- Alstermark B, Lundberg A, Norrsell U, Sybirska E. Integration in descending motor pathways controlling the forelimb in the cat. 9. Differential behavioural defects after spinal cord lesions interrupting defined pathways from higher centres to motoneurones. Exp Brain Res. 1981b;42:299–318. - PubMed
-
- Alstermark B, Lundberg A, Sasaki S. Integration in descending motor pathways controlling the forelimb in the cat. 11. Inhibitory pathways from higher motor centres and forelimb afferents to C3–C4 propriospinal neurones. Exp Brain Res. 1984a;56:293–307. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
