Retrograde transport of Golgi-localized proteins to the ER
- PMID: 9425149
- PMCID: PMC2132605
- DOI: 10.1083/jcb.140.1.1
Retrograde transport of Golgi-localized proteins to the ER
Abstract
The ER is uniquely enriched in chaperones and folding enzymes that facilitate folding and unfolding reactions and ensure that only correctly folded and assembled proteins leave this compartment. Here we address the extent to which proteins that leave the ER and localize to distal sites in the secretory pathway are able to return to the ER folding environment during their lifetime. Retrieval of proteins back to the ER was studied using an assay based on the capacity of the ER to retain misfolded proteins. The lumenal domain of the temperature-sensitive viral glycoprotein VSVGtsO45 was fused to Golgi or plasma membrane targeting domains. At the nonpermissive temperature, newly synthesized fusion proteins misfolded and were retained in the ER, indicating the VSVGtsO45 ectodomain was sufficient for their retention within the ER. At the permissive temperature, the fusion proteins were correctly delivered to the Golgi complex or plasma membrane, indicating the lumenal epitope of VSVGtsO45 also did not interfere with proper targeting of these molecules. Strikingly, Golgi-localized fusion proteins, but not VSVGtsO45 itself, were found to redistribute back to the ER upon a shift to the nonpermissive temperature, where they misfolded and were retained. This occurred over a time period of 15 min-2 h depending on the chimera, and did not require new protein synthesis. Significantly, recycling did not appear to be induced by misfolding of the chimeras within the Golgi complex. This suggested these proteins normally cycle between the Golgi and ER, and while passing through the ER at 40 degrees C become misfolded and retained. The attachment of the thermosensitive VSVGtsO45 lumenal domain to proteins promises to be a useful tool for studying the molecular mechanisms and specificity of retrograde traffic to the ER.
Figures
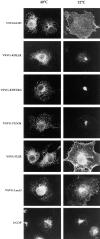




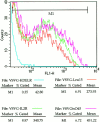



References
-
- Akagi S, Yamamoto A, Yoshimori T, Masaki R, Ogawa R, Tashiro Y. Distribution of protein disulfide isomerase in rat epiphyseal chondrocytes. J Histochem Cytochem. 1989;37:1835–1844. - PubMed
-
- Arar C, Carpentier V, Le Caer JP, Monsigny M, Legrand A, Roche AC. ERGIC-53, a membrane protein of the endoplasmic reticulum-Golgi intermediate compartment, is identical to MR60, an intracellular mannose-specific lectin of myelomonocytic cells. J Biol Chem. 1995;270:3551–3553. - PubMed
-
- Balch WE, Keller DS. ATP-coupled transport of vesicular stomatitis virus G protein. Functional boundaries of secretory compartments. J Biol Chem. 1986;261:14690–14696. - PubMed
-
- Balch WE, Elliott MM, Keller DS. ATP-coupled transport of vesicular stomatitis virus G protein between the endoplasmic reticulum and the Golgi. J Biol Chem. 1986;261:14681–14689. - PubMed
-
- Blum R, Feick P, Puype M, Vandekerckhove J, Klengel R, Nastainczyk W, Schulz I. Tmp21 and p24A, two type I proteins enriched in pancreatic microsomal membranes, are members of a protein family involved in vesicular trafficking. J Biol Chem. 1996;271:17183–17189. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

