Prospore membrane formation defines a developmentally regulated branch of the secretory pathway in yeast
- PMID: 9425151
- PMCID: PMC2132592
- DOI: 10.1083/jcb.140.1.29
Prospore membrane formation defines a developmentally regulated branch of the secretory pathway in yeast
Abstract
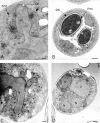
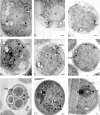
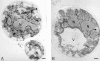
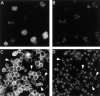
Spore formation in yeast is an unusual form of cell division in which the daughter cells are formed within the mother cell cytoplasm. This division requires the de novo synthesis of a membrane compartment, termed the prospore membrane, which engulfs the daughter nuclei. The effect of mutations in late-acting genes on sporulation was investigated. Mutation of SEC1, SEC4, or SEC8 blocked spore formation, and electron microscopic analysis of the sec4-8 mutant indicated that this inability to produce spores was caused by a failure to form the prospore membrane. The soluble NSF attachment protein 25 (SNAP-25) homologue SEC9, by contrast, was not required for sporulation. The absence of a requirement for SEC9 was shown to be due to the sporulation-specific induction of a second, previously undescribed, SNAP-25 homologue, termed SPO20. These results define a developmentally regulated branch of the secretory pathway and suggest that spore morphogenesis in yeast proceeds by the targeting and fusion of secretory vesicles to form new plasma membranes in the interior of the mother cell. Consistent with this model, the extracellular proteins Gas1p and Cts1p were localized to an internal compartment in sporulating cells. Spore formation in yeast may be a useful model for understanding secretion-driven cell division events in a variety of plant and animal systems.
Figures

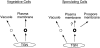
References
-
- Angold RE. The formation of the generative cell in the pollen grain of Emdmyion non-scriptus(L) J Cell Sci. 1968;3:573–578. - PubMed
-
- Brennwald P, Kearns B, Champion K, Keranen S, Bankaitis V, Novick P. Sec9 is a SNAP-25-like component of a yeast SNARE complex that may be the effector of Sec4 function in exocytosis. Cell. 1994;79:245–258. - PubMed
-
- Briza P, Breitenbach M, Ellinger A, Segall J. Isolation of two developmentally regulated genes involved in spore wall maturation in Saccharomyces cerevisiae. . Genes Dev. 1990;4:1775–1789. - PubMed
-
- Byers, B. 1981. Cytology of the yeast life cycle. In The Molecular Biology of the Yeast Saccharomyces: Life Cycle and Inheritance. J.N. Strathern, E.W. Jones, and J.R. Broach, editors. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY. 59–96.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

