Sphingomyelinase treatment induces ATP-independent endocytosis
- PMID: 9425152
- PMCID: PMC2132600
- DOI: 10.1083/jcb.140.1.39
Sphingomyelinase treatment induces ATP-independent endocytosis
Abstract
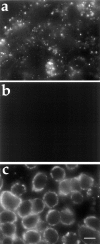
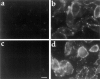
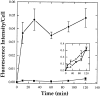
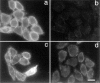
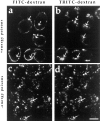
ATP hydrolysis has been regarded as a general requirement for internalization processes in mammalian cells. We found, however, that treatment of ATP-depleted macrophages and fibroblasts with exogenous sphingomyelinase (SMase) rapidly induces formation of numerous vesicles that pinch off from the plasma membrane; the process is complete within 10 min after adding SMase. By electron microscopy, the SMase-induced vesicles are approximately 400 nm in diameter and lack discernible coats. 15-30% of plasma membrane is internalized by SMase treatment, and there is no detectable enrichment of either clathrin or caveolin in these vesicles. When ATP is restored to the cells, the SMase-induced vesicles are able to deliver fluid-phase markers to late endosomes/lysosomes and return recycling receptors, such as transferrin receptors, back to the plasma membrane. We speculate that hydrolysis of sphingomyelin on the plasma membrane causes inward curvature and subsequent fusion to form sealed vesicles. Many cell types express a SMase that can be secreted or delivered to endosomes and lysosomes. The hydrolysis of sphingomyelin by these enzymes is activated by several signaling pathways, and this may lead to formation of vesicles by the process described here.
Figures




References
-
- Allan D, Walklin CM. Endovesiculation of human erythrocytes exposed to sphingomyelinase C: a possible explanation for the enzyme-resistant pool of sphingomyelin. Biochim Biophys Acta. 1988;938:403–410. - PubMed
-
- Bednarek SY, Orci L, Schekman R. Traffic COPs and the formation of vesicle coats. Trends Cell Biol. 1996;6:468–473. - PubMed
-
- Callahan JW, Jones CS, Davidson DJ, Shankaran P. The active site of lysosomal sphingomyelinase: evidence for the involvement of hydrophobic and ionic groups. J Neurosci Res. 1983;10:151–163. - PubMed
-
- Chatterjee S. Neutral sphingomyelinase. Adv Lipid Res. 1993;26:25–48. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

