Low density membranes are associated with RNA-binding proteins and thylakoids in the chloroplast of Chlamydomonas reinhardtii
- PMID: 9425158
- PMCID: PMC2132599
- DOI: 10.1083/jcb.140.1.101
Low density membranes are associated with RNA-binding proteins and thylakoids in the chloroplast of Chlamydomonas reinhardtii
Abstract
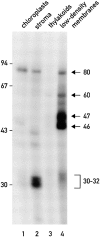
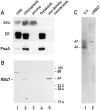
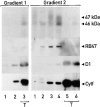
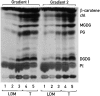
Chloroplast subfractions were tested with a UV cross-linking assay for proteins that bind to the 5' untranslated region of the chloroplast psbC mRNA of the green alga Chlamydomonas reinhardtii. These analyses revealed that RNA-binding proteins of 30-32, 46, 47, 60, and 80 kD are associated with chloroplast membranes. The buoyant density and the acyl lipid composition of these membranes are compatible with their origin being the inner chloroplast envelope membrane. However, unlike previously characterized inner envelope membranes, these membranes are associated with thylakoids. One of the membrane-associated RNA-binding proteins appears to be RB47, which has been reported to be a specific activator of psbA mRNA translation. These results suggest that translation of chloroplast mRNAs encoding thylakoid proteins occurs at either a subfraction of the chloroplast inner envelope membrane or a previously uncharacterized intra-chloroplast compartment, which is physically associated with thylakoids.
Figures



Similar articles
-
A poly(A) binding protein functions in the chloroplast as a message-specific translation factor.Proc Natl Acad Sci U S A. 1998 Mar 3;95(5):2238-43. doi: 10.1073/pnas.95.5.2238. Proc Natl Acad Sci U S A. 1998. PMID: 9482869 Free PMC article.
-
Translation of the chloroplast psbA mRNA requires the nuclear-encoded poly(A)-binding protein, RB47.J Cell Biol. 1998 Jul 27;142(2):435-42. doi: 10.1083/jcb.142.2.435. J Cell Biol. 1998. PMID: 9679142 Free PMC article.
-
Altered mRNA binding activity and decreased translational initiation in a nuclear mutant lacking translation of the chloroplast psbA mRNA.Mol Cell Biol. 1996 Jul;16(7):3560-6. doi: 10.1128/MCB.16.7.3560. Mol Cell Biol. 1996. PMID: 8668172 Free PMC article.
-
Synthesis, membrane insertion and assembly of the chloroplast-encoded D1 protein into photosystem II.FEBS Lett. 2002 Feb 13;512(1-3):13-8. doi: 10.1016/s0014-5793(02)02218-4. FEBS Lett. 2002. PMID: 11852043 Review.
-
Biogenesis and origin of thylakoid membranes.Biochim Biophys Acta. 2001 Dec 12;1541(1-2):91-101. doi: 10.1016/s0167-4889(01)00153-7. Biochim Biophys Acta. 2001. PMID: 11750665 Review.
Cited by
-
Biogenic membranes of the chloroplast in Chlamydomonas reinhardtii.Proc Natl Acad Sci U S A. 2012 Nov 20;109(47):19286-91. doi: 10.1073/pnas.1209860109. Epub 2012 Nov 5. Proc Natl Acad Sci U S A. 2012. PMID: 23129655 Free PMC article.
-
Chloroplast translation regulation.Photosynth Res. 2007 Nov-Dec;94(2-3):359-74. doi: 10.1007/s11120-007-9183-z. Epub 2007 Jul 28. Photosynth Res. 2007. PMID: 17661159 Review.
-
Exploring mechanisms linked to differentiation and function of dimorphic chloroplasts in the single cell C4 species Bienertia sinuspersici.BMC Plant Biol. 2014 Jan 21;14:34. doi: 10.1186/1471-2229-14-34. BMC Plant Biol. 2014. PMID: 24443986 Free PMC article.
-
Interactions of ribosome nascent chain complexes of the chloroplast-encoded D1 thylakoid membrane protein with cpSRP54.EMBO J. 1999 Feb 1;18(3):733-42. doi: 10.1093/emboj/18.3.733. EMBO J. 1999. PMID: 9927433 Free PMC article.
-
Synthesis of the D2 protein of photosystem II in Chlamydomonas is controlled by a high molecular mass complex containing the RNA stabilization factor Nac2 and the translational activator RBP40.Plant Cell. 2007 Nov;19(11):3627-39. doi: 10.1105/tpc.107.051722. Epub 2007 Nov 30. Plant Cell. 2007. PMID: 18055611 Free PMC article.
References
-
- Andreasson E, Svensson P, Weibull C, Albertsson P-A. Separation and characterization of stromal and grana membranes -evidence for heterogeneity in antenna size of both photosystem I and photosystem II. Biochim Biophys Acta. 1988;936:339–350.
-
- Barber J. Membrane surface charges and potentials in relation to photosynthesis. Biochim Biophys Acta. 1980;594:253–308. - PubMed
-
- Block, M.A., A-J. Dorne, J. Joyard, and R. Douce. 1983. Preparation and characterization of membrane fractions enriched in outer and inner envelope membranes from spinach chloroplasts. J. Biol. Chem. 258. 13273–13280. - PubMed
-
- Clemetson JM, Boschetti A, Clemetson KJ. Chloroplast envelope proteins are encoded by the chloroplast genome of Chlamydomonas reinhardtii. . J Biol Chem. 1992;267:19773–19779. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

