Epimorphin functions as a key morphoregulator for mammary epithelial cells
- PMID: 9425164
- PMCID: PMC2132590
- DOI: 10.1083/jcb.140.1.159
Epimorphin functions as a key morphoregulator for mammary epithelial cells
Abstract
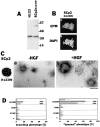
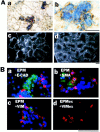
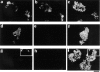
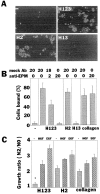
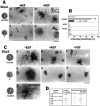
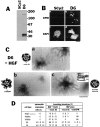
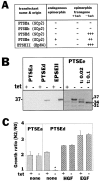
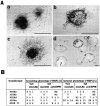
Hepatocyte growth factor (HGF) and EGF have been reported to promote branching morphogenesis of mammary epithelial cells. We now show that it is epimorphin that is primarily responsible for this phenomenon. In vivo, epimorphin was detected in the stromal compartment but not in lumenal epithelial cells of the mammary gland; in culture, however, a subpopulation of mammary epithelial cells produced significant amounts of epimorphin. When epimorphin-expressing epithelial cell clones were cultured in collagen gels they displayed branching morphogenesis in the presence of HGF, EGF, keratinocyte growth factor, or fibroblast growth factor, a process that was inhibited by anti-epimorphin but not anti-HGF antibodies. The branch length, however, was roughly proportional to the ability of the factors to induce growth. Accordingly, epimorphin-negative epithelial cells simply grew in a cluster in response to the growth factors and failed to branch. When recombinant epimorphin was added to these collagen gels, epimorphin-negative cells underwent branching morphogenesis. The mode of action of epimorphin on morphogenesis of the gland, however, was dependent on how it was presented to the mammary cells. If epimorphin was overexpressed in epimorphin-negative epithelial cells under regulation of an inducible promoter or was allowed to coat the surface of each epithelial cell in a nonpolar fashion, the cells formed globular, alveoli-like structures with a large central lumen instead of branching ducts. This process was enhanced also by addition of HGF, EGF, or other growth factors and was inhibited by epimorphin antibodies. These results suggest that epimorphin is the primary morphogen in the mammary gland but that growth factors are necessary to achieve the appropriate cell numbers for the resulting morphogenesis to be visualized.
Figures

References
-
- Bennett MK, Garcia-Arraras JE, Elferink LA, Peterson K, Fleming AM, Hazuka CD, Sheller RH. The syntaxin family of vesicular transport receptors. Cell. 1993;74:863–873. - PubMed
-
- Butt KI, Manabe M, Yaguchi H, Tsuboi R, Ogawa H. Immunolocalization of epimorphin in skin. J Dermatol Sci. 1996;13:193–201. - PubMed
-
- Coleman S, Silberstein GB, Daniel CW. Ductal morphogenesis in the mouse mammary gland: evidence supporting a role for epidermal growth factor. Dev Biol. 1988;127:304–315. - PubMed
-
- Daniel CW, Strickland P, Friedmann Y. Expression and functional role of E- and P-cadherins in mouse mammary ductal morphogenesis and growth. Dev Biol. 1995;169:511–519. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

