Direct and regulated interaction of integrin alphaEbeta7 with E-cadherin
- PMID: 9425167
- PMCID: PMC2132596
- DOI: 10.1083/jcb.140.1.197
Direct and regulated interaction of integrin alphaEbeta7 with E-cadherin
Abstract
The cadherins are a family of homophilic adhesion molecules that play a vital role in the formation of cellular junctions and in tissue morphogenesis. Members of the integrin family are also involved in cell to cell adhesion, but bind heterophilically to immunoglobulin superfamily molecules such as intracellular adhesion molecule (ICAM)-1, vascular cell adhesion molecule (VCAM)-1, or mucosal addressin cell adhesion molecule (MadCAM)-1. Recently, an interaction between epithelial (E-) cadherin and the mucosal lymphocyte integrin, alphaEbeta7, has been proposed. Here, we demonstrate that a human E-cadherin-Fc fusion protein binds directly to soluble recombinant alphaEbeta7, and to alphaEbeta7 solubilized from intraepithelial T lymphocytes. Furthermore, intraepithelial lymphocytes or transfected JY' cells expressing the alphaEbeta7 integrin adhere strongly to purified E-cadherin-Fc coated on plastic, and the adhesion can be inhibited by antibodies to alphaEbeta7 or E-cadherin. The binding of alphaEbeta7 integrin to cadherins is selective since cell adhesion to P-cadherin-Fc through alphaEbeta7 requires >100-fold more fusion protein than to E-cadherin-Fc. Although the structure of the alphaE-chain is unique among integrins, the avidity of alphaEbeta7 for E-cadherin can be regulated by divalent cations or phorbol myristate acetate. Cross-linking of the T cell receptor complex on intraepithelial lymphocytes increases the avidity of alphaEbeta7 for E-cadherin, and may provide a mechanism for the adherence and activation of lymphocytes within the epithelium in the presence of specific foreign antigen. Thus, despite its dissimilarity to known integrin ligands, the specific molecular interaction demonstrated here indicates that E-cadherin is a direct counter receptor for the alphaEbeta7 integrin.
Figures

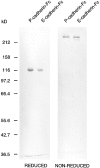






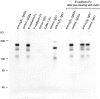
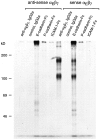

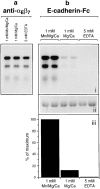
References
-
- Band H, Hochstenbach F, McLean J, Hata S, Krangel MS, Brenner MB. Immunochemical proof that a novel rearranging gene encodes the T cell receptor δ subunit. Science. 1987;238:682–684. - PubMed
-
- Barnstable CJ, Bodmer WF, Brown G, Galfre G, Milstein C, Williams AF, Ziegler A. Production of monoclonal antibodies to group A erythrocytes, HLA and other human cell surface antigens-new tools for genetic analysis. Cell. 1978;14:9–20. - PubMed
-
- Ben-Ze'ev A. Cytoskeletal and adhesion proteins as tumor suppressors. Curr Opin Cell Biol. 1997;9:99–108. - PubMed
-
- Bergelson JM, Hemler ME. Integrin-ligand binding. Do integrins use a ‘MIDAS touch' to grasp an Asp? . Curr Biol. 1995;5:615–617. - PubMed
-
- Blaschuk OW, Sullivan R, David S, Pouliot Y. Identification of a cadherin cell adhesion recognition sequence. Dev Biol. 1990;139:227–229. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

