Identification of p130Cas as a mediator of focal adhesion kinase-promoted cell migration
- PMID: 9425168
- PMCID: PMC2132604
- DOI: 10.1083/jcb.140.1.211
Identification of p130Cas as a mediator of focal adhesion kinase-promoted cell migration
Abstract
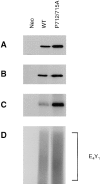
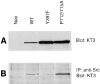
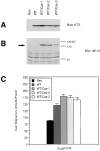
Previously we have demonstrated that focal adhesion kinase (FAK)-promoted migration on fibronectin (FN) by its overexpression in CHO cells is dependent on FAK autophosphorylation at Y397 and subsequent binding of Src to this site. In this report, we have examined the role of FAK association with Grb2 and p130(Cas), two downstream events of the FAK/Src complex that could mediate integrin-stimulated activation of extracellular signal-regulated kinases (Erks). We show that a Y925F FAK mutant was able to promote cell migration as efficiently as FAK and that the transfected FAK demonstrated no detectable association with Grb2 in CHO cells. In contrast, cells expressing a FAK P712/715A mutant demonstrated a level of migration comparable to that of control cells. This mutation did not affect FAK kinase activity, autophosphorylation, or Src association but did significantly reduce p130(Cas) association with FAK. Furthermore, FAK expression in CHO cells increased tyrosine phosphorylation of p130(Cas) and its subsequent binding to several SH2 domains, which depended on both the p130(Cas) binding site and the Src binding site. However, we did not detect increased activation of Erks in cells expressing FAK, and the MEK inhibitor PD98059 did not decrease FAK-promoted cell migration. Finally, we show that coexpression of p130(Cas) further increased cell migration on FN and coexpression of the p130(Cas) SH3 domain alone functioned as a dominant negative mutant and decreased cell migration. Together, these results demonstrate that p130(Cas), but not Grb2, is a mediator of FAK-promoted cell migration and suggest that FAK/ p130(Cas) complex targets downstream pathways other than Erks in mediating FAK-promoted cell migration.
Figures







References
-
- Alessi DR, Cuenda A, Cohen P, Dudley DT, Saltiel AR. PD 098059 is a specific inhibitor of the activation of mitogen-activated protein kinase kinase in vitro and in vivo. J Biol Chem. 1995;270:27489–27494. - PubMed
-
- Cary L, Chang J, Guan J-L. Stimulation of cell migration by overexpression of focal adhesion kinase and its association with Src and Fyn. J Cell Sci. 1996;109:1787–1794. - PubMed
-
- Chan P-Y, Kanner S B, Whitney G, Aruffo A. A transmembrane-anchored chimeric focal adhesion kinase is constitutively activated and phosphorylated at tyrosine residues identical to pp125FAK . J Biol Chem. 1994;269:20567–20574. - PubMed
-
- Chen H-C, Appeddu PA, Parsons JT, Hildebrand JD, Schaller MD, Guan J-L. Interaction of focal adhesion kinase with cytoskeletal protein talin. J Biol Chem. 1995;270:16995–16999. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

