Receptor-independent role of urokinase-type plasminogen activator in pericellular plasmin and matrix metalloproteinase proteolysis during vascular wound healing in mice
- PMID: 9425170
- PMCID: PMC2132607
- DOI: 10.1083/jcb.140.1.233
Receptor-independent role of urokinase-type plasminogen activator in pericellular plasmin and matrix metalloproteinase proteolysis during vascular wound healing in mice
Abstract
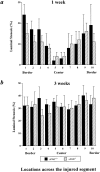
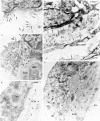
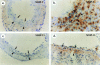
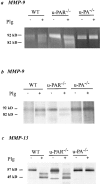
It has been proposed that the urokinase receptor (u-PAR) is essential for the various biological roles of urokinase-type plasminogen activator (u-PA) in vivo, and that smooth muscle cells require u-PA for migration during arterial neointima formation. The present study was undertaken to evaluate the role of u-PAR during this process in mice with targeted disruption of the u-PAR gene (u-PAR-/-). Surprisingly, u-PAR deficiency did not affect arterial neointima formation, neointimal cell accumulation, or migration of smooth muscle cells. Indeed, topographic analysis of arterial wound healing after electric injury revealed that u-PAR-/- smooth muscle cells, originating from the uninjured borders, migrated over a similar distance and at a similar rate into the necrotic center of the wound as wild-type (u-PAR+/+) smooth muscle cells. In addition, u-PAR deficiency did not impair migration of wounded cultured smooth muscle cells in vitro. There were no genotypic differences in reendothelialization of the vascular wound. The minimal role of u-PAR in smooth muscle cell migration was not because of absent expression, since wild-type smooth muscle cells expressed u-PAR mRNA and functional receptor in vitro and in vivo. Pericellular plasmin proteolysis, evaluated by degradation of 125I-labeled fibrin and activation of zymogen matrix metalloproteinases, was similar for u-PAR-/- and u-PAR+/+ cells. Immunoelectron microscopy of injured arteries in vivo revealed that u-PA was bound on the cell surface of u-PAR+/+ cells, whereas it was present in the pericellular space around u-PAR-/- cells. Taken together, these results suggest that binding of u-PA to u-PAR is not required to provide sufficient pericellular u-PA-mediated plasmin proteolysis to allow cellular migration into a vascular wound.
Figures




Similar articles
-
Urokinase but not tissue plasminogen activator mediates arterial neointima formation in mice.Circ Res. 1997 Nov;81(5):829-39. doi: 10.1161/01.res.81.5.829. Circ Res. 1997. PMID: 9351457
-
Deficiency of urokinase-type plasminogen activator-mediated plasmin generation impairs vascular remodeling during hypoxia-induced pulmonary hypertension in mice.Circulation. 2001 Apr 17;103(15):2014-20. doi: 10.1161/01.cir.103.15.2014. Circulation. 2001. PMID: 11306532
-
Impaired arterial neointima formation in mice with disruption of the plasminogen gene.J Clin Invest. 1997 Jan 15;99(2):200-8. doi: 10.1172/JCI119148. J Clin Invest. 1997. PMID: 9005988 Free PMC article.
-
The urokinase receptor and cell migration.Semin Thromb Hemost. 1996;22(6):513-6. doi: 10.1055/s-2007-999053. Semin Thromb Hemost. 1996. PMID: 9122717 Review.
-
Insights in vessel development and vascular disorders using targeted inactivation and transfer of vascular endothelial growth factor, the tissue factor receptor, and the plasminogen system.Ann N Y Acad Sci. 1997 Apr 15;811:191-206. doi: 10.1111/j.1749-6632.1997.tb52002.x. Ann N Y Acad Sci. 1997. PMID: 9186598 Review.
Cited by
-
Fibrin-induced skin fibrosis in mice deficient in tissue plasminogen activator.Am J Pathol. 2005 Sep;167(3):721-32. doi: 10.1016/S0002-9440(10)62046-9. Am J Pathol. 2005. PMID: 16127152 Free PMC article.
-
Potent antitumor activity of a urokinase-activated engineered anthrax toxin.Proc Natl Acad Sci U S A. 2003 Jan 21;100(2):657-62. doi: 10.1073/pnas.0236849100. Epub 2003 Jan 13. Proc Natl Acad Sci U S A. 2003. PMID: 12525700 Free PMC article.
-
Immunomodulatory effects of plasminogen activators on hepatic fibrogenesis.Clin Exp Immunol. 2008 Apr;152(1):163-73. doi: 10.1111/j.1365-2249.2008.03606.x. Epub 2008 Feb 14. Clin Exp Immunol. 2008. PMID: 18279442 Free PMC article.
-
A novel autologous cell-based therapy to promote diabetic wound healing.Ann Surg. 2012 Oct;256(4):560-72. doi: 10.1097/SLA.0b013e31826a9064. Ann Surg. 2012. PMID: 22964729 Free PMC article.
-
Down-regulation of uPA and uPAR by 3,3'-diindolylmethane contributes to the inhibition of cell growth and migration of breast cancer cells.J Cell Biochem. 2009 Nov 1;108(4):916-25. doi: 10.1002/jcb.22323. J Cell Biochem. 2009. Retraction in: J Cell Biochem. 2016 Aug;117(8):1959. doi: 10.1002/jcb.25583. PMID: 19693769 Free PMC article. Retracted.
References
-
- Anichini E, Fibbi G, Pucci M, Caldini R, Chevanne M, Del Rosso M. Production of second messengers following chemotactic and mitogenic urokinase-receptor interaction in human fibroblasts and mouse fibroblasts transfected with human urokinase receptor. Exp Cell Res. 1994;213:438–448. - PubMed
-
- Behrendt N, Ronne E, Dano K. The structure and function of the urokinase receptor, a membrane protein governing plasminogen activation on the cell surface. Biol Chem Hoppe Seyler. 1995;376:269–279. - PubMed
-
- Bendeck MP, Zempo N, Clowes AW, Galardy RE, Reidy MA. Smooth muscle cell migration and matrix metalloproteinase expression after arterial injury in the rat. Circ Res. 1994;75:539–545. - PubMed
-
- Blasi F, Conese M, Moller LB, Pedersen N, Cavallaro U, Cubellis MV, Fazioli F, Hernandez-Marrero L, Limongi P, Munoz-Canoves P, et al. The urokinase receptor: structure, regulation and inhibitor- mediated internalization. Fibrinolysis. 1994;8:182–188.
-
- Bugge TH, Suh TT, Flick MJ, Daugherty CC, Romer J, Solberg H, Ellis V, Dano K, Degen JL. The receptor for urokinase-type plasminogen activator is not essential for mouse development or fertility. J Biol Chem. 1995;270:16886–16894. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

