Interleukin 10 secretion and impaired effector function of major histocompatibility complex class II-restricted T cells anergized in vivo
- PMID: 9432975
- PMCID: PMC2212096
- DOI: 10.1084/jem.187.2.177
Interleukin 10 secretion and impaired effector function of major histocompatibility complex class II-restricted T cells anergized in vivo
Abstract
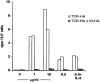
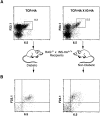
Continuous antigenic stimulation in vivo can result in the generation of so-called "anergic" CD4(+) or CD8(+) T cells that fail to proliferate upon antigenic stimulation and fail to develop cytolytic effector functions. Here we show that class II major histocompatibility complex-restricted T cells specific for influenza hemagglutinin (HA) that become anergic in mice expressing HA under control of the immunoglobulin kappa promoter exhibit an impaired effector function in causing diabetes in vivo, as compared to their naive counterparts, when transferred into immunodeficient recipients expressing HA under the control of the insulin promoter. Furthermore, HA-specific T cells anergized in vivo contain higher levels of interleukin (IL)-4 messenger RNA (mRNA) than naive and recently activated T cells with the same specificity and more than a 100-fold higher levels of IL-10 mRNA. The higher expression of the IL-10 gene is also evident at the protein level. These findings raise the interesting possibility that T cells rendered anergic in vivo have in fact become regulatory T cells that may influence neighboring immune responses through the release of IL-10.
Figures





References
-
- Kisielow P, Bluthmann H, Staerz UD, Steinmetz M, von Boehmer H. Tolerance in T cell receptor transgenic mice involves deletion of nonmature CD4+8+thymocytes. Nature. 1988;333:742–746. - PubMed
-
- Kappler JW, Roehm N, Marrack P. T cell tolerance by clonal elimination in the thymus. Cell. 1987;49:273–280. - PubMed
-
- Rocha B, von Boehmer H. Peripheral selection of the T cell repertoire. Science. 1991;251:1225–1227. - PubMed
-
- Moskophidis D, Laine E, Zinkernagel RM. Peripheral clonal deletion of antiviral memory CD8+T cells. Eur J Immunol. 1993;23:3306–3311. - PubMed
-
- Kearney ER, Pape KA, Loh DY, Jenkins MK. Visualization of peptide-specific T cell immunity and peripheral tolerance induction in vivo. J Exp Med. 1994;1:327–339. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

