Residue-specific bioincorporation of non-natural, biologically active amino acids into proteins as possible drug carriers: structure and stability of the per-thiaproline mutant of annexin V
- PMID: 9435213
- PMCID: PMC18441
- DOI: 10.1073/pnas.95.2.455
Residue-specific bioincorporation of non-natural, biologically active amino acids into proteins as possible drug carriers: structure and stability of the per-thiaproline mutant of annexin V
Abstract
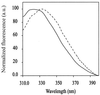
Residue-specific bioincorporation of 1,3-thiazolidine-4-carboxylic acid [thiaproline, Pro(S)], a non-natural amino acid analog of proline, into human recombinant annexin V was achieved with a proline-auxotrophic Escherichia coli strain by fermentation procedures in minimal medium. Quantitative replacement of proline with thiaproline was confirmed by mass-spectrometric, amino acid, and x-ray crystallographic analyses. The wild-type protein and its per-Pro(S) mutant were found to crystallize isomorphously and to show identical three-dimensional structures in crystals. In solution the dichroic properties of the wild-type and per-Pro(S) protein confirmed nearly identical overall folds. From thermal denaturation experiments, however, a reduced Tm (-4.5 K) value was determined whereas the van't Hoff enthalpy and entropy were not significantly affected. Therefore, protein mutants containing bioactive amino acid analogs like thiaproline at multiple sites would be expected to fully retain their functional properties, including immunogenicity, and thus could serve as promising vehicles for targeted drug delivery.
Figures
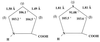





References
-
- Noren C J, Anthony-Cahill S J, Griffith M C, Shultz P G. Science. 1989;244:182–188. - PubMed
-
- Budisa N, Steipe B, Demange P, Eckerskorn C, Kellermann J, Huber R. Eur J Biochem. 1995;230:788–796. - PubMed
-
- Budisa N, Karnbrock W, Steinbacher S, Humm A, Prade L, Neuefeind T, Moroder L, Huber R. J Mol Biol. 1997;270:616–623. - PubMed
-
- Kern D, Schutkowski M, Drakenberg T. J Am Chem Soc. 1997;119:8403–8408.
-
- Sheehan J C, Yang D-D H. J Am Chem Soc. 1958;80:1158–1164.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

