Uric acid, a natural scavenger of peroxynitrite, in experimental allergic encephalomyelitis and multiple sclerosis
- PMID: 9435251
- PMCID: PMC18479
- DOI: 10.1073/pnas.95.2.675
Uric acid, a natural scavenger of peroxynitrite, in experimental allergic encephalomyelitis and multiple sclerosis
Abstract
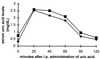
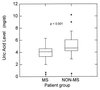
Uric acid, the naturally occurring product of purine metabolism, is a strong peroxynitrite scavenger, as demonstrated by the capacity to bind peroxynitrite but not nitric oxide (NO) produced by lipopolysaccharide-stimulated cells of a mouse monocyte line. In this study, we used uric acid to treat experimental allergic encephalomyelitis (EAE) in the PLSJL strain of mice, which develop a chronic form of the disease with remissions and exacerbations. Uric acid administration was found to have strong therapeutic effects in a dose-dependent fashion. A regimen of four daily doses of 500 mg/kg uric acid was required to promote long-term survival regardless of whether treatment was initiated before or after the clinical symptoms of EAE had appeared. The requirement for multiple doses is likely to be caused by the rapid clearance of uric acid in mice which, unlike humans, metabolize uric acid a step further to allantoin. Uric acid treatment also was found to diminish clinical signs of a disease resembling EAE in interferon-gamma receptor knockout mice. A possible association between multiple sclerosis (MS), the disease on which EAE is modeled, and uric acid is supported by the finding that patients with MS have significantly lower levels of serum uric acid than controls. In addition, statistical evaluation of more than 20 million patient records for the incidence of MS and gout (hyperuricemic) revealed that the two diseases are almost mutually exclusive, raising the possibility that hyperuricemia may protect against MS.
Figures






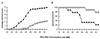
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

