TcpP protein is a positive regulator of virulence gene expression in Vibrio cholerae
- PMID: 9435261
- PMCID: PMC18489
- DOI: 10.1073/pnas.95.2.730
TcpP protein is a positive regulator of virulence gene expression in Vibrio cholerae
Abstract
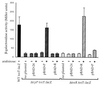
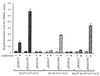
The production of several virulence factors in Vibrio cholerae O1, including cholera toxin and the pilus colonization factor TCP (toxin-coregulated pilus), is strongly influenced by environmental conditions. To specifically identify membrane proteins involved in these signal transduction events, we examined a transposon library of V. cholerae generated by Tnbla mutagenesis for cells that produce TCP when grown under various nonpermissive conditions. To select for TCP-producing cells we used the recently described bacteriophage CTX phi-Kan, which uses TCP as its receptor and carries a gene encoding resistance to kanamycin. Among the isolated mutants was a transposon insertion in a gene homologous to nqrB from Vibrio alginolyticus, which encodes a subunit of a Na(+)-translocating NADH:ubiquinone oxidoreductase, and tcpI, encoding a chemo-receptor previously implicated in the negative regulation of TCP production. A third transposon mutant had an insertion in tcpP, which is in an operon with tcpH, a known positive regulator of TCP production. However, TcpP was shown to be essential for TCP production in V. cholerae, as a tcpP-deletion strain was deficient in pili production. The amino-terminal region of TcpP shows sequence homology to the DNA-binding domains of several regulatory proteins, including ToxR from V. cholerae and PsaE from Yersinia pestis. Like ToxR, TcpP activates transcription of the toxT gene, an essential activator of tcp operon transcription. Furthermore, TcpH, with its large periplasmic domain and inner membrane anchor, has a structure similar to that of ToxS and was shown to enhance the activity of TcpP. We propose that TcpP/TcpH constitute a pair of regulatory proteins functionally similar to ToxR/ToxS and PsaE/PsaF that are required for toxT transcription in V. cholerae.
Figures
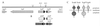

References
-
- DiRita V J. In: Two-component Signal Transduction. Hoch J A, Silhavy T J, editors. Washington, DC: Am. Soc. Microbiol.; 1995. pp. 351–365.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

