Cellular and developmental control of O2 homeostasis by hypoxia-inducible factor 1 alpha
- PMID: 9436976
- PMCID: PMC316445
- DOI: 10.1101/gad.12.2.149
Cellular and developmental control of O2 homeostasis by hypoxia-inducible factor 1 alpha
Abstract
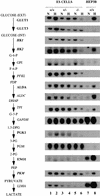
Hypoxia is an essential developmental and physiological stimulus that plays a key role in the pathophysiology of cancer, heart attack, stroke, and other major causes of mortality. Hypoxia-inducible factor 1 (HIF-1) is the only known mammalian transcription factor expressed uniquely in response to physiologically relevant levels of hypoxia. We now report that in Hif1a-/- embryonic stem cells that did not express the O2-regulated HIF-1alpha subunit, levels of mRNAs encoding glucose transporters and glycolytic enzymes were reduced, and cellular proliferation was impaired. Vascular endothelial growth factor mRNA expression was also markedly decreased in hypoxic Hif1a-/- embryonic stem cells and cystic embryoid bodies. Complete deficiency of HIF-1alpha resulted in developmental arrest and lethality by E11 of Hif1a-/- embryos that manifested neural tube defects, cardiovascular malformations, and marked cell death within the cephalic mesenchyme. In Hif1a+/+ embryos, HIF-1alpha expression increased between E8.5 and E9.5, coincident with the onset of developmental defects and cell death in Hif1a-/- embryos. These results demonstrate that HIF-1alpha is a master regulator of cellular and developmental O2 homeostasis.
Figures









References
-
- Andersen B, Rosenfeld MG. Pit-1 determines cell types during development of the anterior pituitary gland: A model for transcriptional regulation of cell phenotypes in mammalian organogenesis. J Biol Chem. 1994;269:29335–29338. - PubMed
-
- Ausubel FM, Brent R, Kingston RE, Moore DD, Seidman JG, Smith JA, Struhl K, Albright LM, Coen D, Varki MA, Janssen K. Current protocols in molecular biology. New York, NY: John Wiley and Sons; 1994.
-
- Bradley A. Production and analysis of chimaeric mice. In: Robertson EJ, editor. Teratocarcinomas and embryonic stem cells: A practical approach. 1st ed. Oxford, UK: IRL Press; 1987. pp. 113–152.
-
- Carmeliet P, Ferreira V, Breier G, Pollefeyt S, Kieckens L, Gertesenstein M, Fahrig M, Vandenhoeck A, Harpal K, Eberhardt C, Declercq C, Pawling J, Moons L, Collen D, Risau W, Nagy A. Abnormal blood vessel development and lethality in embryos lacking a single VEGF allele. Nature. 1996;380:435–439. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
