Functional and regulatory interactions between Hox and extradenticle genes
- PMID: 9436985
- PMCID: PMC316439
- DOI: 10.1101/gad.12.2.261
Functional and regulatory interactions between Hox and extradenticle genes
Abstract
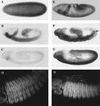
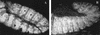
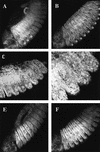
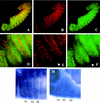
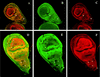
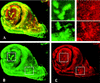
The homeobox gene extradenticle (exd) acts as a cofactor of Hox function both in Drosophila and vertebrates. It has been shown that the distribution of the Exd protein is developmentally regulated at the post-translational level; in the regions where exd is not functional Exd is present only in the cell cytoplasm, whereas it accumulates in the nuclei of cells requiring exd function. We show that the subcellular localization of Exd is regulated by the BX-C genes and that each BX-C gene can prevent or reduce nuclear translocation of Exd to different extents. In spite of this negative regulation, two BX-C genes, Ultrabithorax and abdominal-A, require exd activity for their maintenance and function. We propose that mutual interactions between Exd and BX-C proteins ensure the correct amounts of interacting molecules. As the Hoxd10 gene has the same properties as Drosophila BX-C genes, we suggest that the control mechanism of subcellular distribution of Exd found in Drosophila probably operates in other organisms as well.
Figures



References
-
- Aspland SE, White RAH. Nucleocytoplsmic localisation of extradenticle protein is spatially regulated throughout development in Drosophila. Development. 1997;124:741–747. - PubMed
-
- Brand AH, Perrimon N. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development. 1993;118:401–415. - PubMed
-
- Casanova J, Sanchez-Herrero E, Morata G. Identification and characterisation of a parasegment specific regulatory element of the Abdominal-B gene of Drosophila. Cell. 1986;47:627–636. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
