DRhoGEF2 encodes a member of the Dbl family of oncogenes and controls cell shape changes during gastrulation in Drosophila
- PMID: 9436986
- PMCID: PMC316438
- DOI: 10.1101/gad.12.2.274
DRhoGEF2 encodes a member of the Dbl family of oncogenes and controls cell shape changes during gastrulation in Drosophila
Abstract
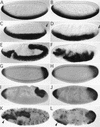
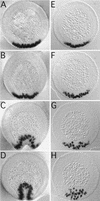
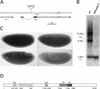
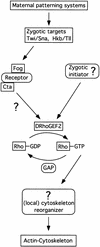
We have identified a gene, DRhoGEF2, which encodes a putative guanine nucleotide exchange factor belonging to the Dbl family of oncogenes. DRhoGEF2 function is essential for the coordination of cell shape changes during gastrulation. In the absence of maternal DRhoGEF2 gene activity, mesodermal and endodermal primordia fail to invaginate. The phenotype seen in DRhoGEF2 mutants is more severe than the defects associated with mutations in two previously identified gastrulation genes, folded gastrulation and concertina, suggesting that DRhoGEF2 acts in a signaling pathway independent of these genes. Expression of dominant-negative DRhoA during gastrulation results in phenocopies of the DRhoGEF2 mutant, suggesting that a signaling cascade involving DRhoGEF2 and the small GTPase DRhoA is responsible for the regulation of cell shape changes during early Drosophila morphogenesis.
Figures



Similar articles
-
The Rho GTPase and a putative RhoGEF mediate a signaling pathway for the cell shape changes in Drosophila gastrulation.Cell. 1997 Dec 26;91(7):905-15. doi: 10.1016/s0092-8674(00)80482-1. Cell. 1997. PMID: 9428514
-
A Rho GTPase signaling pathway is used reiteratively in epithelial folding and potentially selects the outcome of Rho activation.Curr Biol. 2004 Oct 26;14(20):1822-6. doi: 10.1016/j.cub.2004.09.080. Curr Biol. 2004. PMID: 15498489
-
Hyperactivation of the folded gastrulation pathway induces specific cell shape changes.Development. 1998 Feb;125(4):589-97. doi: 10.1242/dev.125.4.589. Development. 1998. PMID: 9435280
-
Regulation and function of the terminal gap gene huckebein in the Drosophila blastoderm.Int J Dev Biol. 1996 Feb;40(1):157-65. Int J Dev Biol. 1996. PMID: 8735925 Review.
-
Drosophila morphogenesis: orchestrating cell rearrangements.Curr Biol. 1998 Nov 19;8(23):R853-5. doi: 10.1016/s0960-9822(07)00530-1. Curr Biol. 1998. PMID: 9822573 Review.
Cited by
-
Untying the Gordian knot of cytokinesis. Role of small G proteins and their regulators.J Cell Biol. 2000 Mar 6;148(5):843-8. doi: 10.1083/jcb.148.5.843. J Cell Biol. 2000. PMID: 10704435 Free PMC article. Review. No abstract available.
-
The conserved WW-domain binding sites in Dystroglycan C-terminus are essential but partially redundant for Dystroglycan function.BMC Dev Biol. 2009 Feb 27;9:18. doi: 10.1186/1471-213X-9-18. BMC Dev Biol. 2009. PMID: 19250553 Free PMC article.
-
Hox targets and cellular functions.Scientifica (Cairo). 2013;2013:738257. doi: 10.1155/2013/738257. Epub 2013 Dec 30. Scientifica (Cairo). 2013. PMID: 24490109 Free PMC article.
-
Intrakinetochore localization and essential functional domains of Drosophila Spc105.EMBO J. 2009 Aug 19;28(16):2374-86. doi: 10.1038/emboj.2009.188. Epub 2009 Jul 9. EMBO J. 2009. PMID: 19590494 Free PMC article.
-
The Rho Guanine Nucleotide Exchange Factor DRhoGEF2 Is a Genetic Modifier of the PI3K Pathway in Drosophila.PLoS One. 2016 Mar 25;11(3):e0152259. doi: 10.1371/journal.pone.0152259. eCollection 2016. PLoS One. 2016. PMID: 27015411 Free PMC article.
References
-
- Ahmed S, Kozma R, Lee J, Monfries C, Harden N, Lim L. The cysteine-rich domain of human proteins, neuronal chimaerin, protein kinase C and diacylglycerol kinase binds zinc. Evidence for the involvement of a zinc-dependent structure in phorbol ester binding. Biochem J. 1991;280:233–241. - PMC - PubMed
-
- Bell RM, Burns DJ. Lipid activation of protein kinase C. J Biol Chem. 1991;266:4661–4664. - PubMed
-
- Brand AH, Perrimon N. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development. 1993;118:401–415. - PubMed
-
- Brönner G, Chu-LaGraff Q, Doe CQ, Cohen B, Weigel D, Taubert H, Jäckle H. Sp1/egr-like zinc-finger protein required for endoderm specification and germ-layer formation in Drosophila. Nature. 1994;369:664–668. - PubMed
-
- Brown NH, Kafatos FC. Functional cDNA libraries from Drosophila embryos. J Mol Biol. 1988;203:425–437. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
