Plasma membrane localization of G alpha z requires two signals
- PMID: 9436987
- PMCID: PMC25209
- DOI: 10.1091/mbc.9.1.1
Plasma membrane localization of G alpha z requires two signals
Abstract
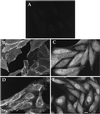
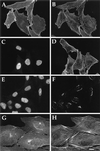
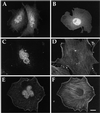
Three covalent attachments anchor heterotrimeric G proteins to cellular membranes: the alpha subunits are myristoylated and/or palmitoylated, whereas the gamma chain is prenylated. Despite the essential role of these modifications in membrane attachment, it is not clear how they cooperate to specify G protein localization at the plasma membrane, where the G protein relays signals from cell surface receptors to intracellular effector molecules. To explore this question, we studied the effects of mutations that prevent myristoylation and/or palmitoylation of an epitope-labeled alpha subunit, alpha z. Wild-type alpha z (alpha z-WT) localizes specifically at the plasma membrane. A mutant that incorporates only myristate is mistargeted to intracellular membranes, in addition to the plasma membrane, but transduces hormonal signals as well as does alpha z-WT. Removal of the myristoylation site produced a mutant alpha z that is located in the cytosol, is not efficiently palmitoylated, and does not relay the hormonal signal. Coexpression of beta gamma with this myristoylation defective mutant transfers it to the plasma membrane, promotes its palmitoylation, and enables it to transmit hormonal signals. Pulse-chase experiments show that the palmitate attached to this myristoylation-defective mutant turns over much more rapidly than does palmitate on alpha z-WT, and that the rate of turnover is further accelerated by receptor activation. In contrast, receptor activation does not increase the slow rate of palmitate turnover on alpha z-WT. Together these results suggest that myristate and beta gamma promote stable association with membranes not only by providing hydrophobicity, but also by stabilizing attachment of palmitate. Moreover, palmitoylation confers on alpha z specific localization at the plasma membrane.
Figures


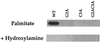



Similar articles
-
Gbetagamma and palmitate target newly synthesized Galphaz to the plasma membrane.J Biol Chem. 1999 Jun 25;274(26):18793-800. doi: 10.1074/jbc.274.26.18793. J Biol Chem. 1999. PMID: 10373496
-
Localization of a peripheral membrane protein: Gbetagamma targets Galpha(Z).Proc Natl Acad Sci U S A. 2000 Feb 1;97(3):1085-90. doi: 10.1073/pnas.97.3.1085. Proc Natl Acad Sci U S A. 2000. PMID: 10655488 Free PMC article.
-
Palmitoylation of a G protein alpha i subunit requires membrane localization not myristoylation.J Biol Chem. 1994 Dec 9;269(49):30898-903. J Biol Chem. 1994. PMID: 7983022
-
Lipid modifications and membrane targeting of G alpha.Biol Signals Recept. 1998 Mar-Apr;7(2):125-35. doi: 10.1159/000014538. Biol Signals Recept. 1998. PMID: 9629464 Review.
-
Fatty acylation of proteins: new insights into membrane targeting of myristoylated and palmitoylated proteins.Biochim Biophys Acta. 1999 Aug 12;1451(1):1-16. doi: 10.1016/s0167-4889(99)00075-0. Biochim Biophys Acta. 1999. PMID: 10446384 Review.
Cited by
-
Huntingtin interacting protein 14 is an oncogenic human protein: palmitoyl acyltransferase.Oncogene. 2004 Dec 9;23(57):9230-7. doi: 10.1038/sj.onc.1208171. Oncogene. 2004. PMID: 15489887 Free PMC article.
-
Except in every detail: comparing and contrasting G-protein signaling in Saccharomyces cerevisiae and Schizosaccharomyces pombe.Eukaryot Cell. 2005 Mar;4(3):495-503. doi: 10.1128/EC.4.3.495-503.2005. Eukaryot Cell. 2005. PMID: 15755912 Free PMC article. Review. No abstract available.
-
From fat to fire: The lipid-inflammasome connection.Immunol Rev. 2025 Jan;329(1):e13403. doi: 10.1111/imr.13403. Epub 2024 Sep 27. Immunol Rev. 2025. PMID: 39327931 Free PMC article. Review.
-
Specificity of plasma membrane targeting by the rous sarcoma virus gag protein.J Virol. 2003 Jan;77(1):470-80. doi: 10.1128/jvi.77.1.470-480.2003. J Virol. 2003. PMID: 12477852 Free PMC article.
-
Membrane trafficking of heterotrimeric G proteins via the endoplasmic reticulum and Golgi.Mol Biol Cell. 2002 Sep;13(9):3294-302. doi: 10.1091/mbc.e02-02-0095. Mol Biol Cell. 2002. PMID: 12221133 Free PMC article.
References
-
- Berthiaume L, Resh MD. Biochemical characterization of a palmitoyl acyltransferase activity that palmitoylates myristoylated proteins. J Biol Chem. 1995;270:22399–22405. - PubMed
-
- Bhatnagar RS, Gordon JI. Understanding covalent modifications of proteins by lipids: where cell biology and biophysics mingle. Trends Cell Biol. 1997;7:14–20. - PubMed
-
- Bigay J, Faurobert E, Franco M, Chabre M. Roles of lipid modifications of transducin subunits in their GDP-dependent association and membrane binding. Biochemistry. 1994;33:14081–14090. - PubMed
-
- Boman AL, Kahn RA. Arf proteins: the membrane traffic police? Trends Biochem Sci. 1995;20:147–150. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

