Developmental expression of spectrins in rat skeletal muscle
- PMID: 9436990
- PMCID: PMC25216
- DOI: 10.1091/mbc.9.1.47
Developmental expression of spectrins in rat skeletal muscle
Abstract
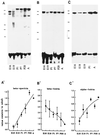
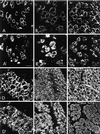
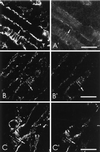
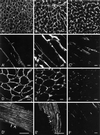
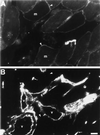
Skeletal muscle contains spectrin (or spectrin I) and fodrin (or spectrin II), members of the spectrin supergene family. We used isoform-specific antibodies and cDNA probes to investigate the molecular forms, developmental expression, and subcellular localization of the spectrins in skeletal muscle of the rat. We report that beta-spectrin (betaI) replaces beta-fodrin (betaII) at the sarcolemma as skeletal muscle fibers develop. As a result, adult muscle fibers contain only alpha-fodrin (alphaII) and the muscle isoform of beta-spectrin (betaISigma2). By contrast, other types of cells present in skeletal muscle tissue, including blood vessels and nerves, contain only alpha- and beta-fodrin. During late embryogenesis and early postnatal development, skeletal muscle fibers contain a previously unknown form of spectrin complex, consisting of alpha-fodrin, beta-fodrin, and the muscle isoform of beta-spectrin. These complexes associate with the sarcolemma to form linear membrane skeletal structures that otherwise resemble the structures found in the adult. Our results suggest that the spectrin-based membrane skeleton of muscle fibers can exist in three distinct states during development.
Figures



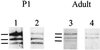

Similar articles
-
Na,K-ATPase in skeletal muscle: two populations of beta-spectrin control localization in the sarcolemma but not partitioning between the sarcolemma and the transverse tubules.J Cell Sci. 2001 Feb;114(Pt 4):751-62. doi: 10.1242/jcs.114.4.751. J Cell Sci. 2001. PMID: 11171381
-
Two populations of beta-spectrin in rat skeletal muscle.Cell Motil Cytoskeleton. 1997;37(1):7-19. doi: 10.1002/(SICI)1097-0169(1997)37:1<7::AID-CM2>3.0.CO;2-7. Cell Motil Cytoskeleton. 1997. PMID: 9142435
-
Spectrins in developing rat hippocampal cells.Brain Res Dev Brain Res. 2001 Jul 23;129(1):81-93. doi: 10.1016/s0165-3806(01)00160-2. Brain Res Dev Brain Res. 2001. PMID: 11454415
-
The spectrin super-family.Biol Cell. 1991;71(3):249-54. Biol Cell. 1991. PMID: 1933022 Review.
-
The role of βII spectrin in cardiac health and disease.Life Sci. 2018 Jan 1;192:278-285. doi: 10.1016/j.lfs.2017.11.009. Epub 2017 Nov 9. Life Sci. 2018. PMID: 29128512 Free PMC article. Review.
Cited by
-
Differential regulation of apoptosis in slow and fast twitch muscles of aged female F344BN rats.Age (Dordr). 2015;37(2):30. doi: 10.1007/s11357-015-9767-z. Epub 2015 Mar 28. Age (Dordr). 2015. PMID: 25813803 Free PMC article.
-
Characteristics of primary Sjögren's syndrome-associated peripheral nervous system lesions.J Neurol. 2023 Nov;270(11):5527-5535. doi: 10.1007/s00415-023-11883-z. Epub 2023 Jul 31. J Neurol. 2023. PMID: 37523064
-
Ankyrin-B interactions with spectrin and dynactin-4 are required for dystrophin-based protection of skeletal muscle from exercise injury.J Biol Chem. 2011 Mar 4;286(9):7370-8. doi: 10.1074/jbc.M110.187831. Epub 2010 Dec 25. J Biol Chem. 2011. PMID: 21186323 Free PMC article.
-
Neuromuscular electrical stimulation promotes development in mice of mature human muscle from immortalized human myoblasts.Skelet Muscle. 2016 Feb 27;6:4. doi: 10.1186/s13395-016-0078-6. eCollection 2016. Skelet Muscle. 2016. PMID: 26925213 Free PMC article.
-
Role of an alternatively spliced form of alphaII-spectrin in localization of connexin 43 in cardiomyocytes and regulation by stress-activated protein kinase.J Mol Cell Cardiol. 2007 Mar;42(3):572-81. doi: 10.1016/j.yjmcc.2006.11.018. Epub 2007 Feb 5. J Mol Cell Cardiol. 2007. PMID: 17276456 Free PMC article.
References
-
- Beck KA, Nelson WJ. The spectrin-based membrane skeleton as a membrane protein-sorting machine. Am J Physiol. 1996;270:C1263–1270. - PubMed
-
- Bennett V. Protein involved in membrane-cytoskeleton association in human erythrocytes: spectrin, ankyrin, and band 3. Methods Enzymol. 1983;96:313–324. - PubMed
-
- Bennett V, Baines AJ, Davis J. Purification of brain analogs of red blood cell membrane skeletal proteins: ankyrin, protein 4.1 (synapsin), spectrin, and spectrin subunits. Methods Enzymol. 1986;134:55–69. - PubMed
-
- Bennett V, Gilligan DM. The spectrin-based membrane skeleton and micron-scale organization of the plasma membrane. Annu Rev Cell Biol. 1993;9:27–66. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

