Negative regulation of Cdc18 DNA replication protein by Cdc2
- PMID: 9436991
- PMCID: PMC25219
- DOI: 10.1091/mbc.9.1.63
Negative regulation of Cdc18 DNA replication protein by Cdc2
Abstract
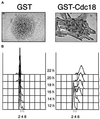
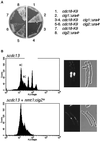
Fission yeast Cdc18, a homologue of Cdc6 in budding yeast and metazoans, is periodically expressed during the S phase and required for activation of replication origins. Cdc18 overexpression induces DNA rereplication without mitosis, as does elimination of Cdc2-Cdc13 kinase during G2 phase. These findings suggest that illegitimate activation of origins may be prevented through inhibition of Cdc18 by Cdc2. Consistent with this hypothesis, we report that Cdc18 interacts with Cdc2 in association with Cdc13 and Cig2 B-type cyclins in vivo. Cdc18 is phosphorylated by the associated Cdc2 in vitro. Mutation of a single phosphorylation site, T104A, activates Cdc18 in the rereplication assay. The cdc18-K9 mutation is suppressed by a cig2 mutation, providing genetic evidence that Cdc2-Cig2 kinase inhibits Cdc18. Moreover, constitutive expression of Cig2 prevents rereplication in cells lacking Cdc13. These findings identify Cdc18 as a key target of Cdc2-Cdc13 and Cdc2-Cig2 kinases in the mechanism that limits chromosomal DNA replication to once per cell cycle.
Figures




Similar articles
-
Functionally homologous DNA replication genes in fission and budding yeast.J Cell Sci. 1999 Jul;112 ( Pt 14):2381-90. doi: 10.1242/jcs.112.14.2381. J Cell Sci. 1999. PMID: 10381393
-
Rum1 and Cdc18 link inhibition of cyclin-dependent kinase to the initiation of DNA replication in Schizosaccharomyces pombe.Genes Dev. 1996 Mar 1;10(5):541-52. doi: 10.1101/gad.10.5.541. Genes Dev. 1996. PMID: 8598285
-
Interaction of the S phase regulator cdc18 with cyclin-dependent kinase in fission yeast.Proc Natl Acad Sci U S A. 1997 Jun 10;94(12):6142-7. doi: 10.1073/pnas.94.12.6142. Proc Natl Acad Sci U S A. 1997. PMID: 9177184 Free PMC article.
-
Regulation of G1 progression in fission yeast by the rum1+ gene product.Prog Cell Cycle Res. 1996;2:29-35. doi: 10.1007/978-1-4615-5873-6_3. Prog Cell Cycle Res. 1996. PMID: 9552380 Review.
-
Cyclins of the fission yeast Schizosaccharomyces pombe.Semin Cell Biol. 1995 Apr;6(2):73-8. doi: 10.1016/1043-4682(95)90003-9. Semin Cell Biol. 1995. PMID: 7548845 Review.
Cited by
-
Cdc18/CDC6 activates the Rad3-dependent checkpoint in the fission yeast.Nucleic Acids Res. 2007;35(16):5323-37. doi: 10.1093/nar/gkm527. Epub 2007 Aug 8. Nucleic Acids Res. 2007. PMID: 17690116 Free PMC article.
-
Assembly of a complex containing Cdc45p, replication protein A, and Mcm2p at replication origins controlled by S-phase cyclin-dependent kinases and Cdc7p-Dbf4p kinase.Mol Cell Biol. 2000 May;20(9):3086-96. doi: 10.1128/MCB.20.9.3086-3096.2000. Mol Cell Biol. 2000. PMID: 10757793 Free PMC article.
-
PR48, a novel regulatory subunit of protein phosphatase 2A, interacts with Cdc6 and modulates DNA replication in human cells.Mol Cell Biol. 2000 Feb;20(3):1021-9. doi: 10.1128/MCB.20.3.1021-1029.2000. Mol Cell Biol. 2000. PMID: 10629059 Free PMC article.
-
Identification and characterization of Saccharomyces cerevisiae Cdc6 DNA-binding properties.Mol Biol Cell. 2000 May;11(5):1673-85. doi: 10.1091/mbc.11.5.1673. Mol Biol Cell. 2000. PMID: 10793143 Free PMC article.
-
Chromatin binding of the fission yeast replication factor mcm4 occurs during anaphase and requires ORC and cdc18.EMBO J. 2000 Apr 3;19(7):1681-90. doi: 10.1093/emboj/19.7.1681. EMBO J. 2000. PMID: 10747035 Free PMC article.
References
-
- Alfa C, Fantes P, Hyams J, McLeod M, Warbrick E. Experiments with Fission Yeast. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1993.
-
- Boyle WJ, Van Der Geer P, Hunter T. Phosphopeptide mapping and phosphoamino acid analysis by two-dimensional separation on thin layer cellulose plates. Methods Enzymol. 1991;210:110–149. - PubMed
-
- Broek D, Bartlett R, Crawford K, Nurse P. Involvement of p34cdc2 in establishing the dependency of S phase on mitosis. Nature. 1991;349:388–393. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

