Tyrosine phosphorylation regulates cell cycle-dependent nuclear localization of Cdc48p
- PMID: 9436996
- PMCID: PMC25228
- DOI: 10.1091/mbc.9.1.131
Tyrosine phosphorylation regulates cell cycle-dependent nuclear localization of Cdc48p
Abstract
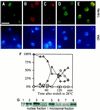
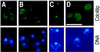
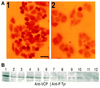
Cdc48p from Saccharomyces cerevisiae and its highly conserved mammalian homologue VCP (valosin-containing protein) are ATPases with essential functions in cell division and homotypic fusion of endoplasmic reticulum vesicles. Both are mainly attached to the endoplasmic reticulum, but relocalize in a cell cycle-dependent manner: Cdc48p enters the nucleus during late G1; VCP aggregates at the centrosome during mitosis. The nuclear import signal sequence of Cdc48p was localized near the amino terminus and its function demonstrated by mutagenesis. The nuclear import is regulated by a cell cycle-dependent phosphorylation of a tyrosine residue near the carboxy terminus. Two-hybrid studies indicate that the phosphorylation results in a conformational change of the protein, exposing the nuclear import signal sequence previously masked by a stretch of acidic residues.
Figures



Similar articles
-
Identification of the regions of porcine VCP preventing its function in Saccharomyces cerevisiae.Gene. 1997 Dec 19;204(1-2):145-51. doi: 10.1016/s0378-1119(97)00535-0. Gene. 1997. PMID: 9434177
-
AAA-ATPase p97/Cdc48p, a cytosolic chaperone required for endoplasmic reticulum-associated protein degradation.Mol Cell Biol. 2002 Jan;22(2):626-34. doi: 10.1128/MCB.22.2.626-634.2002. Mol Cell Biol. 2002. PMID: 11756557 Free PMC article.
-
VCP, the mammalian homolog of cdc48, is tyrosine phosphorylated in response to T cell antigen receptor activation.EMBO J. 1992 Oct;11(10):3533-40. doi: 10.1002/j.1460-2075.1992.tb05436.x. EMBO J. 1992. PMID: 1382975 Free PMC article.
-
ATP-bound form of the D1 AAA domain inhibits an essential function of Cdc48p/p97.Biochem Cell Biol. 2010 Feb;88(1):109-17. doi: 10.1139/o09-116. Biochem Cell Biol. 2010. PMID: 20130684 Review.
-
Cdc48p is UBX-linked to ER ubiquitin ligases.Trends Biochem Sci. 2006 Jan;31(1):24-5. doi: 10.1016/j.tibs.2005.11.004. Epub 2005 Nov 28. Trends Biochem Sci. 2006. PMID: 16316751 Review.
Cited by
-
Studies on peptide:N-glycanase-p97 interaction suggest that p97 phosphorylation modulates endoplasmic reticulum-associated degradation.Proc Natl Acad Sci U S A. 2007 May 22;104(21):8785-90. doi: 10.1073/pnas.0702966104. Epub 2007 May 11. Proc Natl Acad Sci U S A. 2007. PMID: 17496150 Free PMC article.
-
Autophagy Dysfunction in ALS: from Transport to Protein Degradation.J Mol Neurosci. 2022 Jul;72(7):1456-1481. doi: 10.1007/s12031-022-02029-3. Epub 2022 Jun 16. J Mol Neurosci. 2022. PMID: 35708843 Free PMC article. Review.
-
The localization and phosphorylation of p47 are important for Golgi disassembly-assembly during the cell cycle.J Cell Biol. 2003 Jun 23;161(6):1067-79. doi: 10.1083/jcb.200303048. Epub 2003 Jun 16. J Cell Biol. 2003. PMID: 12810701 Free PMC article.
-
A complex of mammalian ufd1 and npl4 links the AAA-ATPase, p97, to ubiquitin and nuclear transport pathways.EMBO J. 2000 May 15;19(10):2181-92. doi: 10.1093/emboj/19.10.2181. EMBO J. 2000. PMID: 10811609 Free PMC article.
-
Nucleocytoplasmic shuttling of valosin-containing protein (VCP/p97) regulated by its N domain and C-terminal region.Biochim Biophys Acta. 2015 Jan;1853(1):222-32. doi: 10.1016/j.bbamcr.2014.10.019. Epub 2014 Oct 30. Biochim Biophys Acta. 2015. PMID: 25447673 Free PMC article.
References
-
- Acharya U, Jacobs R, Peters JM, Watson N, Farquhar MG, Malhotra V. The formation of Golgi stacks from vesiculated Golgi membranes requires two distinct fusion events. Cell. 1995;82:895–904. - PubMed
-
- Bartel P, Chien C, Sternglanz R, Fields S. Elimination of false positives that arise in using the two-hybrid system. BioTechniques. 1993;14:920–924. - PubMed
-
- Broach JR, Li Y-Y, Wu L-CC, Jayaram M. Vectors for high-level, inducible expression of cloned genes in yeast. In: Inouye M, editor. Experimental Manipulation of Gene Expression. London: Academic Press; 1983. pp. 83–117.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

