Molecular characterization of a novel, widespread nuclear protein that colocalizes with spliceosome components
- PMID: 9436997
- PMCID: PMC25229
- DOI: 10.1091/mbc.9.1.143
Molecular characterization of a novel, widespread nuclear protein that colocalizes with spliceosome components
Abstract
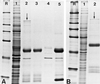
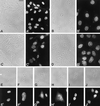
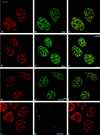
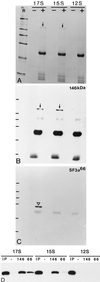
We report the identification and molecular characterization of a novel type of constitutive nuclear protein that is present in diverse vertebrate species, from Xenopus laevis to human. The cDNA-deduced amino acid sequence of the Xenopus protein defines a polypeptide of a calculated mass of 146.2 kDa and a isoelectric point of 6.8, with a conspicuous domain enriched in the dipeptide TP (threonine-proline) near its amino terminus. Immunolocalization studies in cultured cells and tissues sections of different origin revealed an exclusive nuclear localization of the protein. The protein is diffusely distributed in the nucleoplasm but concentrated in nuclear speckles, which represent a subnuclear compartment enriched in small nuclear ribonucleoprotein particles and other splicing factors, as confirmed by colocalization with certain splicing factors and Sm proteins. During mitosis, when transcription and splicing are downregulated, the protein is released from the nuclear speckles and transiently dispersed throughout the cytoplasm. Biochemical experiments have shown that the protein is recovered in a approximately 12S complex, and gel filtration studies confirm that the protein is part of a large particle. Immunoprecipitation and Western blot analysis of chromatographic fractions enriched in human U2 small nuclear ribonucleoprotein particles of distinct sizes (12S, 15S, and 17S), reflecting their variable association with splicing factors SF3a and SF3b, strongly suggests that the 146-kDa protein reported here is a constituent of the SF3b complex.
Figures





Similar articles
-
Characterization of novel SF3b and 17S U2 snRNP proteins, including a human Prp5p homologue and an SF3b DEAD-box protein.EMBO J. 2002 Sep 16;21(18):4978-88. doi: 10.1093/emboj/cdf480. EMBO J. 2002. PMID: 12234937 Free PMC article.
-
Combined biochemical and electron microscopic analyses reveal the architecture of the mammalian U2 snRNP.J Cell Biol. 1999 Jun 28;145(7):1355-68. doi: 10.1083/jcb.145.7.1355. J Cell Biol. 1999. PMID: 10385517 Free PMC article.
-
Interaction of mammalian splicing factor SF3a with U2 snRNP and relation of its 60-kD subunit to yeast PRP9.Science. 1993 Oct 1;262(5130):102-5. doi: 10.1126/science.8211112. Science. 1993. PMID: 8211112
-
Identification and characterization of a novel kind of nuclear protein occurring free in the nucleoplasm and in ribonucleoprotein structures of the "speckle" type.Eur J Cell Biol. 1998 Apr;75(4):295-308. doi: 10.1016/S0171-9335(98)80063-0. Eur J Cell Biol. 1998. PMID: 9628316
-
Structure-function analysis of the U2 snRNP-associated splicing factor SF3a.Biochem Soc Trans. 2005 Jun;33(Pt 3):439-42. doi: 10.1042/BST0330439. Biochem Soc Trans. 2005. PMID: 15916536 Review.
Cited by
-
Changes in the Honeybee Antioxidant System after 12 h of Exposure to Electromagnetic Field Frequency of 50 Hz and Variable Intensity.Insects. 2020 Oct 18;11(10):713. doi: 10.3390/insects11100713. Insects. 2020. PMID: 33081029 Free PMC article.
-
U2 snRNP binds intronless histone pre-mRNAs to facilitate U7-snRNP-dependent 3' end formation.Mol Cell. 2007 Oct 26;28(2):240-52. doi: 10.1016/j.molcel.2007.09.026. Mol Cell. 2007. PMID: 17964263 Free PMC article.
-
Characterization of novel SF3b and 17S U2 snRNP proteins, including a human Prp5p homologue and an SF3b DEAD-box protein.EMBO J. 2002 Sep 16;21(18):4978-88. doi: 10.1093/emboj/cdf480. EMBO J. 2002. PMID: 12234937 Free PMC article.
-
Mammalian polycomb-mediated repression of Hox genes requires the essential spliceosomal protein Sf3b1.Genes Dev. 2005 Mar 1;19(5):536-41. doi: 10.1101/gad.1284605. Genes Dev. 2005. PMID: 15741318 Free PMC article.
-
Domains in human splicing factors SF3a60 and SF3a66 required for binding to SF3a120, assembly of the 17S U2 snRNP, and prespliceosome formation.Mol Cell Biol. 2001 Oct;21(19):6406-17. doi: 10.1128/MCB.21.19.6406-6417.2001. Mol Cell Biol. 2001. PMID: 11533230 Free PMC article.
References
-
- Ankenbauer T, Kleinschmidt JA, Walsh MJ, Weiner OH, Franke WW. Identification of a widespread nuclear actin-binding protein. Nature. 1989;342:822–825. - PubMed
-
- Baserga SJ, Steitz JA. The diverse world of small ribonucleoproteins. In: Gesteland RF, Atkins JF, editors. The RNA World. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1993. pp. 359–381.
-
- Bennett M, Reed R. Correspondence between a mammalian spliceosome component and an essential yeast splicing factor. Science. 1993;262:105–108. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources

