Transport and turnover of microtubules in frog neurons depend on the pattern of axonal growth
- PMID: 9437004
- PMCID: PMC6792771
- DOI: 10.1523/JNEUROSCI.18-03-00821.1998
Transport and turnover of microtubules in frog neurons depend on the pattern of axonal growth
Abstract
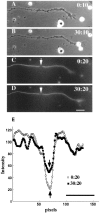
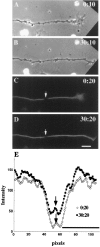
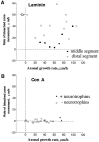
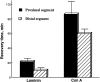
The transport of axonal microtubules in growing neurites has been a controversial issue because of clear but conflicting results obtained with fluorescence-marking techniques. We have attempted to resolve the discordance via analysis of the relationship between apparent microtubule translocation and cell adhesion. Neuronal cultures were prepared from Xenopus embryos 1 d after injection of Cy3-conjugated tubulin into one of the blastomeres of two-cell-stage embryos. Anterograde translocation of axonal microtubules was observed in neurons cultured on a laminin-coated surface, in agreement with previously published data for Xenopus embryonic neurons. However, when neuronal cultures were prepared on a concanavalin A-treated surface, the axonal microtubules were stationary, as reported for all other neurons investigated previously. Neuronal cultures prepared on laminin- and concanavalin A-coated surfaces also demonstrated dramatic differences in the pattern of axonal growth, dynamics of axonal microtubules, and response to brefeldin A treatment. Our findings suggest that transport and dynamics of axonal microtubules may be directly affected by the mechanical tension produced by growth cone activity.
Figures



References
-
- Ahmad FJ, Baas PW. Microtubules released from the neuronal centrosomes are transported into the axon. J Cell Sci. 1995;108:2761–2769. - PubMed
-
- Baas PW. Microtubules and axonal growth. Curr Opin Cell Biol. 1997;9:29–36. - PubMed
-
- Bamburg JR, Bray D, Chapman K. Assembly of microtubules at the tip of growing axons. Nature. 1986;321:788–790. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
