An open rectifier potassium channel with two pore domains in tandem cloned from rat cerebellum
- PMID: 9437008
- PMCID: PMC6792778
- DOI: 10.1523/JNEUROSCI.18-03-00868.1998
An open rectifier potassium channel with two pore domains in tandem cloned from rat cerebellum
Abstract
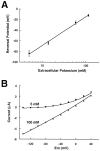
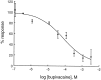
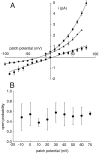
Tandem pore domain K+ channels represent a new family of ion channels involved in the control of background membrane conductances. We report the structural and functional properties of a TWIK-related acid-sensitive K+ channel (rTASK), a new member of this family cloned from rat cerebellum. The salient features of the primary amino acid sequence include four putative transmembrane domains and, unlike other cloned tandem pore domain channels, a PDZ (postsynaptic density protein, disk-large, zo-1) binding sequence at the C terminal. rTASK has distant overall homology to a putative Caenorhabditis elegans K+ channel and to the mammalian clones TREK-1 and TWIK-1. rTASK expression is most abundant in rat heart, lung, and brain. When exogenously expressed in Xenopus oocytes, rTASK currents activate instantaneously, are noninactivating, and are not gated by voltage. Because rTASK currents satisfy the Goldman-Hodgkin-Katz current equation for an open channel, rTASK can be classified an open rectifier. Activation of protein kinase A produces inhibition of rTASK, whereas activation of protein kinase C has no effect. rTASK currents were inhibited by extracellular acidity. rTASK currents also were inhibited by Zn2+ (IC50 = 175 microM), the local anesthetic bupivacaine (IC50 = 68 microM), and the anti-convulsant phenytoin ( approximately 50% inhibition at 200 microM). By demonstrating open rectification and open probability independent of voltage, we have established that rTASK is a baseline potassium channel.
Figures






Similar articles
-
Polymodal Mechanism for TWIK-Related K+ Channel Inhibition by Local Anesthetic.Anesth Analg. 2019 Oct;129(4):973-982. doi: 10.1213/ANE.0000000000004216. Anesth Analg. 2019. PMID: 31124840
-
Local anesthetic inhibition of baseline potassium channels with two pore domains in tandem.Anesthesiology. 1999 Apr;90(4):1092-102. doi: 10.1097/00000542-199904000-00024. Anesthesiology. 1999. PMID: 10201682
-
A novel two-pore domain K+ channel, TRESK, is localized in the spinal cord.J Biol Chem. 2003 Jul 25;278(30):27406-12. doi: 10.1074/jbc.M206810200. Epub 2003 May 17. J Biol Chem. 2003. PMID: 12754259
-
TWIK-2, a new weak inward rectifying member of the tandem pore domain potassium channel family.J Biol Chem. 1999 Mar 19;274(12):7887-92. doi: 10.1074/jbc.274.12.7887. J Biol Chem. 1999. PMID: 10075682
-
Two aspects of the inward rectification mechanism. Effects of cytoplasmic blockers and extracellular K+ on the inward rectifier K+ channel.Jpn Heart J. 1996 Sep;37(5):631-41. doi: 10.1536/ihj.37.631. Jpn Heart J. 1996. PMID: 8973376 Review.
Cited by
-
SUMOylation silences heterodimeric TASK potassium channels containing K2P1 subunits in cerebellar granule neurons.Sci Signal. 2012 Nov 20;5(251):ra84. doi: 10.1126/scisignal.2003431. Sci Signal. 2012. PMID: 23169818 Free PMC article.
-
Mutants of a temperature-sensitive two-P domain potassium channel.J Neurosci. 2000 Oct 15;20(20):7517-24. doi: 10.1523/JNEUROSCI.20-20-07517.2000. J Neurosci. 2000. PMID: 11027209 Free PMC article.
-
Motoneurons express heteromeric TWIK-related acid-sensitive K+ (TASK) channels containing TASK-1 (KCNK3) and TASK-3 (KCNK9) subunits.J Neurosci. 2004 Jul 28;24(30):6693-702. doi: 10.1523/JNEUROSCI.1408-04.2004. J Neurosci. 2004. PMID: 15282272 Free PMC article.
-
The role of acid-sensitive two-pore domain potassium channels in cardiac electrophysiology: focus on arrhythmias.Pflugers Arch. 2015 May;467(5):1055-67. doi: 10.1007/s00424-014-1637-5. Epub 2014 Nov 19. Pflugers Arch. 2015. PMID: 25404566 Review.
-
Functional and molecular identification of a TASK-1 potassium channel regulating chloride secretion through CFTR channels in the shark rectal gland: implications for cystic fibrosis.Am J Physiol Cell Physiol. 2016 Dec 1;311(6):C884-C894. doi: 10.1152/ajpcell.00030.2016. Epub 2016 Sep 21. Am J Physiol Cell Physiol. 2016. PMID: 27653983 Free PMC article.
References
-
- Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. - PubMed
-
- Andrews RJ, Bringas JR, Alonzo G. Cerebrospinal fluid pH and PCO2 rapidly follow arterial blood pH and PCO2 with changes in ventilation. Neurosurgery. 1994;34:466–470. - PubMed
-
- Assaf SY, Chung SH. Release of endogenous Zn2+ from brain tissue during activity. Nature. 1984;308:734–736. - PubMed
-
- Brenman JE, Chao DS, Gee SH, McGee AW, Craven SE, Santillano DR, Wu Z, Huang F, Xia H, Peters MF, Froehner SC, Bredt DS. Interaction of nitric oxide synthase with the postsynaptic density protein PSD-95 and alpha1-syntrophin mediated by PDZ domains. Cell. 1996;84:757–767. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
