The Ca2+ channel beta3 subunit differentially modulates G-protein sensitivity of alpha1A and alpha1B Ca2+ channels
- PMID: 9437009
- PMCID: PMC6792773
- DOI: 10.1523/JNEUROSCI.18-03-00878.1998
The Ca2+ channel beta3 subunit differentially modulates G-protein sensitivity of alpha1A and alpha1B Ca2+ channels
Abstract
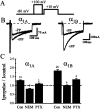
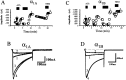
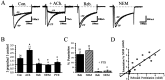
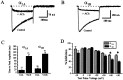
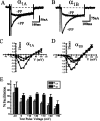
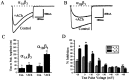
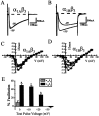
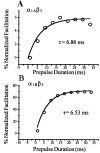
We have shown previously that the Ca2+ channel beta3 subunit is capable of modulating tonic G-protein inhibition of alpha1A and alpha1B Ca2+ channels expressed in oocytes. Here we determine the modulatory effect of the Ca2+ channel beta3 subunit on M2 muscarinic receptor-activated G-protein inhibition and whether the beta3 subunit modulates the G-protein sensitivity of alpha1A and alpha1B currents equivalently. To compare the relative inhibition by muscarinic activation, we have used successive ACh applications to remove the large tonic inhibition of these channels. We show that the resulting rebound potentiation results entirely from the loss of tonic G-protein inhibition; although the currents are temporarily relieved of tonic inhibition, they are still capable of undergoing inhibition through the muscarinic pathway. Using this rebound protocol, we demonstrate that the inhibition of peak current amplitude produced by M2 receptor activation is similar for alpha1A and alpha1B calcium currents. However, the contribution of the voltage-dependent component of inhibition, characterized by reduced inhibition at very depolarized voltage steps and the relief of inhibition by depolarizing prepulses, was slightly greater for the alpha1B current than for the alpha1A current. After co-expression of the beta3 subunit, the sensitivity to M2 receptor-induced G-protein inhibition was reduced for both alpha1A and alpha1B currents; however, the reduction was significantly greater for alpha1A currents. Additionally, the difference in the voltage dependence of inhibition of alpha1A and alpha1B currents was heightened after co-expression of the Ca2+ channel beta3 subunit. Such differential modulation of sensitivity to G-protein modulation may be important for fine tuning release in neurons that contain both of these Ca2+ channels.
Figures
Similar articles
-
Ca2+ channel beta3 subunit enhances voltage-dependent relief of G-protein inhibition induced by muscarinic receptor activation and Gbetagamma.J Neurosci. 1998 Jul 1;18(13):4883-90. doi: 10.1523/JNEUROSCI.18-13-04883.1998. J Neurosci. 1998. PMID: 9634554 Free PMC article.
-
Determinants of the G protein-dependent opioid modulation of neuronal calcium channels.Proc Natl Acad Sci U S A. 1996 Feb 20;93(4):1486-91. doi: 10.1073/pnas.93.4.1486. Proc Natl Acad Sci U S A. 1996. PMID: 8643659 Free PMC article.
-
Gating properties of GIRK channels activated by Galpha(o)- and Galpha(i)-coupled muscarinic m2 receptors in Xenopus oocytes: the role of receptor precoupling in RGS modulation.J Physiol. 2002 Dec 1;545(2):355-73. doi: 10.1113/jphysiol.2002.032151. J Physiol. 2002. PMID: 12456817 Free PMC article.
-
Modulation of ion channels by somatostatin and acetylcholine.Prog Neurobiol. 1992;38(2):203-30. doi: 10.1016/0301-0082(92)90040-l. Prog Neurobiol. 1992. PMID: 1372125 Review.
-
Ca2+ channels as integrators of G protein-mediated signaling in neurons.Mol Pharmacol. 2004 Nov;66(5):1071-6. doi: 10.1124/mol.104.002261. Epub 2004 Jul 21. Mol Pharmacol. 2004. PMID: 15269290 Review.
Cited by
-
Mutations in high-voltage-activated calcium channel genes stimulate low-voltage-activated currents in mouse thalamic relay neurons.J Neurosci. 2002 Aug 1;22(15):6362-71. doi: 10.1523/JNEUROSCI.22-15-06362.2002. J Neurosci. 2002. PMID: 12151514 Free PMC article.
-
The ß subunit of voltage-gated Ca2+ channels.Physiol Rev. 2010 Oct;90(4):1461-506. doi: 10.1152/physrev.00057.2009. Physiol Rev. 2010. PMID: 20959621 Free PMC article. Review.
-
Intracellular Na+ inhibits voltage-dependent N-type Ca2+ channels by a G protein betagamma subunit-dependent mechanism.J Physiol. 2004 Apr 1;556(Pt 1):121-34. doi: 10.1113/jphysiol.2003.056168. Epub 2004 Jan 23. J Physiol. 2004. PMID: 14742725 Free PMC article.
-
Interaction between G proteins and accessory subunits in the regulation of 1B calcium channels in Xenopus oocytes.J Physiol. 2000 Sep 15;527 Pt 3(Pt 3):419-32. doi: 10.1111/j.1469-7793.2000.t01-1-00419.x. J Physiol. 2000. PMID: 10990530 Free PMC article.
-
Protein kinase C is involved in M1-muscarinic receptor-mediated facilitation of L-type Ca2+ channels in neurons of the major pelvic ganglion of the adult male rat.Neurochem Res. 2001 Sep;26(8-9):933-42. doi: 10.1023/a:1012332500946. Neurochem Res. 2001. PMID: 11699945
References
-
- Bean BP. Neurotransmitter inhibition of neuronal calcium currents by changes in channel voltage dependence. Nature. 1989;340:153–156. - PubMed
-
- Beech DJ, Bernheim L, Hille B. Pertussis toxin and voltage dependence distinguish multiple pathways modulating calcium channels of rat sympathetic neurons. Neuron. 1992;8:97–106. - PubMed
-
- Bernheim L, Beech DJ, Hille B. A diffusible second messenger mediates one of the pathways coupling receptors to Ca2+ channels in rat sympathetic neurons. Neuron. 1991;6:859–867. - PubMed
-
- Birnbaumer L, Campbell K, Catterall WA, Harpold MM, Hofman F, Horne WA, Mori Y, Schwartz A, Snutch TP, Tanabe T, Tsien RW. Matters arising: the naming of voltage gated calcium channels. Neuron. 1994;13:505–506. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
