A role for L-type calcium channels in developmental regulation of transmitter phenotype in primary sensory neurons
- PMID: 9437025
- PMCID: PMC6792782
- DOI: 10.1523/JNEUROSCI.18-03-01047.1998
A role for L-type calcium channels in developmental regulation of transmitter phenotype in primary sensory neurons
Abstract
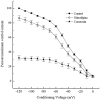
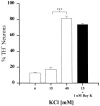
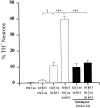
To examine the influence of activity-dependent cues on differentiation of primary afferent neurons, we investigated the short- and long-term effects of depolarization and calcium influx on expression of transmitter traits in sensory ganglion cell cultures. We focused on expression of tyrosine hydroxylase (TH), a marker for dopaminergic neurons, in developing petrosal ganglion (PG), nodose ganglion, and dorsal root ganglion neurons grown in the presence or absence of depolarizing concentrations of KCl. Exposure to 40 mM KCl increased the proportion of TH-immunoreactive neurons in all three ganglia in a developmentally regulated manner that corresponded to the temporal pattern of dopaminergic expression in vivo. PG neurons, for example, were most responsive to elevated KCl on embryonic day 16.5 (E16.5), the age at which the dopaminergic phenotype is first detectable in vivo. However, KCl was relatively ineffective at increasing TH expression in neonatal PG, indicating a critical period for induction of this phenotype by depolarization. Detailed analysis of TH induction in PG neurons demonstrated that, although N-type calcium channels carried the majority of the high voltage-activated calcium current, only L-type calcium channel blockade inhibited the effect of elevated KCl. Further studies revealed that after removal of high KCl, neurons remained sensitized to subsequent stimulation for >1 week. Specifically, cultures exposed to KCl beginning on E16.5 (the conditioning stimulus), then returned to control medium, and subsequently re-exposed to elevated KCl after 9 d (the test stimulus) contained fourfold more TH-positive neurons than did cultures exposed to the test stimulus alone. Moreover, blockade of L-type calcium channels during the conditioning stimulus completely abolished long-term potentiation of the TH response to elevated KCl. These findings demonstrate a novel role for L-type calcium channels in activity-dependent plasticity of transmitter expression in sensory neurons and indicate that exposure to depolarizing stimuli during early development may alter neuronal response properties at later ages.
Figures








References
-
- Altman J, Bayer SA. Development of the cranial nerve ganglia and related nuclei in the rat. Adv Anat Embryol Cell Biol. 1982;74:1–87. - PubMed
-
- Bading H, Ginty DD, Greenberg ME. Regulation of gene expression in hippocampal neurons by distinct calcium signaling pathways. Science. 1993;260:181–186. - PubMed
-
- Baker H, Farbman AI. Olfactory afferent regulation of the dopamine phenotype in the fetal rat olfactory system. Neuroscience. 1993;52:115–134. - PubMed
-
- Bisgard GE, Neubauer JA. Peripheral and central effects of hypoxia. In: Dempsey JA, Pack AI, editors. Lung biology in health and disease: regulation of breathing. Dekker; New York: 1995. pp. 617–668.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
