The role of visual experience in the development of columns in cat visual cortex
- PMID: 9438851
- PMCID: PMC2453000
- DOI: 10.1126/science.279.5350.566
The role of visual experience in the development of columns in cat visual cortex
Abstract
The role of experience in the development of the cerebral cortex has long been controversial. Patterned visual experience in the cat begins when the eyes open about a week after birth. Cortical maps for orientation and ocular dominance in the primary visual cortex of cats were found to be present by 2 weeks. Early pattern vision appeared unimportant because these cortical maps developed identically until nearly 3 weeks of age, whether or not the eyes were open. The naïve maps were powerfully dominated by the contralateral eye, and experience was needed for responses to the other eye to become strong, a process unlikely to be strictly Hebbian. With continued visual deprivation, responses to both eyes deteriorated, with a time course parallel to the well-known critical period of cortical plasticity. The basic structure of cortical maps is therefore innate, but experience is essential for specific features of these maps, as well as for maintaining the responsiveness and selectivity of cortical neurons.
Figures

References
-
- Wiesel T. Nature. 1982;299:583. - PubMed
- Movshon JA, Kiorpes L. In: Development of Sensory Systems in Mammals. Coleman JR, editor. Wiley; New York: 1990. pp. 155–202.
-
- Bonhoeffer T, Grinvald A. Brain Mapping: The Methodology. Academic Press; New York: 1996. pp. 75–97. Optical intrinsic signal responses were measured as in. and in (14). Normalization by response to stimulation with a blank screen was used in all figures and analysis.
-
-
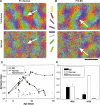
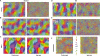
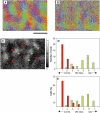
Microelectrode studies have found single units that are selective for orientation at P8 (30) and we have seen clear orientation maps as early as P12
-
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

