A randomized trial comparing cisplatin plus 5-fluorouracil with or without levamisole in operable gastric cancer
- PMID: 9439150
- PMCID: PMC4531987
- DOI: 10.3904/kjim.1997.12.2.155
A randomized trial comparing cisplatin plus 5-fluorouracil with or without levamisole in operable gastric cancer
Abstract
Objectives: To determine the effectiveness and toxicity when levamisole was added to the adjuvant combination chemotherapy in patients with operable gastric cancer.
Methods: After en bloc resection of gastric cancer without gross or microscopic evidence of residual disease from April 1991 to December 1992, 100 patients were randomized to 6 months of 5-fluorouracil 1,000 mg/m2/day administered as continuous infusion for 5 days, cisplatin 60 mg/m2/day as intravenous infusion for 1 day with or without levamisole (50 mg every eight hours P.O for a period of three days every 2 weeks for 6 months). This chemotherapy treatment was begun within 2 to 4 weeks after the surgery. The chemotherapy consisted of discrete 5-day courses administered at 4-weeks intervals. All 100 patients are assessable.
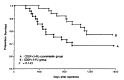
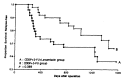
Results: The fifty patients were assigned to each treatment group. There was no statistical difference and no bias in the distribution of characteristics of the 100 evaluable patients between the two groups. A total of 274 courses of treatment were given in the levamisole group and 260 courses of treatment in non-levamisole group. Eleven patients in each group did not finish planned 6 courses of treatment mainly due to non-compliance. At median follow up of 39 months, 32 patients relapsed 19 in the levamisole group and 13 in the non-levamisole group (p = 0.284). Twenty five patients died of relapsed diseases, 15 in the levamisole group and 10 in the non-levamisole group. The levamisole group tended to show more risk of overall death rate and recurrence than the non-levamisole group. However, this result was not statistically significant at 3 years. The treatment was well tolerated in both treatment groups. The grade 2-3 toxicities were nausea/ vomiting (levamisole, non-levamisole group; 31.7%, 29.3% of treatment courses respectively), diarrhea (7.6%, 8.4%), mucositis (11.6%, 12.3%), and leukopenia (9.8%, 9.6%).
Conclusion: Levamisole had negative effects on disease-free survival and overall survival when added to adjuvant combination chemotherapy of cisplatin and 5-fluorouracil in patients with operable gastric cancer. Both treatment arms were generally well tolerated and the toxicity profile was similar with or without levamisole.
Figures
References
-
- Renoux G. The general immunopharmacology of levamisole. Drugs. 1980;20:89–99. - PubMed
-
- Stevenson Henry C, Green Ira, Hamilton J Michael, Calabro Barbara A, Parkinso R. Levamisole:Known Effects on the Immune System, Clinical Results and Future Applications to the Treatment of Cancer. J Clin Oncol. 1991;9:2052–2066. - PubMed
-
- Amery WK. Final results of a multicenter placebo-controlled levamisole study of resectable lung cancer. Cancer Treat Rep. 1978;62:1677–1683. - PubMed
-
- Kamby C, Rose C, Ejlertsen B, Andersen J, Brikler NE, Rytter LRytter, Andersen KW, Zedeler K. Adjuvant Systemic Treatment and the Pattern of Recurrences in Patients with Breast Cancer. Eur J Cancer Clin Oncol. 1988;24:439–447. - PubMed
-
- Mortel Charles G, Fleming Thomas R, Macdonald John S, Haller Daniel G, Laurie John A, Tangen Catherine M, Ungerleider James S, Emerson William A, Tormey Douglass C, Glick John H, Veeder Michael H, Mailliard James A. Fluorouracil plus Levamisole as Effective Adjuvant Therapy after Resection of Stage III Colon Carcinoma: A Final Report. Ann Intern Med. 1995;122:321–326. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous