Functional analysis of the two-gene lysis system of the pneumococcal phage Cp-1 in homologous and heterologous host cells
- PMID: 9440507
- PMCID: PMC106873
- DOI: 10.1128/JB.180.2.210-217.1998
Functional analysis of the two-gene lysis system of the pneumococcal phage Cp-1 in homologous and heterologous host cells
Abstract
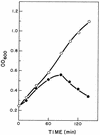
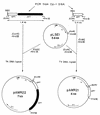
The two lysis genes cph1 and cpl1 of the Streptococcus pneumoniae bacteriophage Cp-1 coding for holin and lysozyme, respectively, have been cloned and expressed in Escherichia coli. Synthesis of the Cph1 holin resulted in bacterial cell death but not lysis. The cph1 gene was able to complement a lambda Sam mutation in the nonsuppressing E. coli HB101 strain to produce phage progeny, suggesting that the holins encoded by both phage genes have analogous functions and that the pneumococcal holin induces a nonspecific lesion in the cytoplasmic membrane. Concomitant expression of both holin and lysin of Cp-1 in E. coli resulted in cell lysis, apparently due to the ability of the Cpl1 lysozyme to hydrolyze the peptidoglycan layer of this bacterium. The functional analysis of the cph1 and cpl1 genes cloned in a pneumococcal mutant with a complete deletion of the lytA gene, which codes for the S. pneumoniae main autolysin, provided the first direct evidence that, in this gram-positive-bacterium system, the Cpl1 endolysin is released to its murein substrate through the activity of the Cph1 holin. Demonstration of holin function was achieved by proving the release of pneumolysin to the periplasmic fraction, which strongly suggested that the holin produces a lesion in the pneumococcal membrane.
Figures





Similar articles
-
Identification of a holin encoded by the Streptomyces aureofaciens phage micro1/6; functional analysis in Escherichia coli system.Folia Microbiol (Praha). 2004;49(6):679-84. doi: 10.1007/BF02931549. Folia Microbiol (Praha). 2004. PMID: 15881403
-
The two-step lysis system of pneumococcal bacteriophage EJ-1 is functional in gram-negative bacteria: triggering of the major pneumococcal autolysin in Escherichia coli.Mol Microbiol. 1996 Feb;19(4):667-81. doi: 10.1046/j.1365-2958.1996.399929.x. Mol Microbiol. 1996. PMID: 8820638
-
Lytic action of cloned pneumococcal phage lysis genes in Streptococcus pneumoniae.FEMS Microbiol Lett. 1993 Mar 15;108(1):87-92. doi: 10.1016/0378-1097(93)90492-k. FEMS Microbiol Lett. 1993. PMID: 8472929
-
Bacteriophages of Streptococcus pneumoniae: a molecular approach.Microb Drug Resist. 1997 Summer;3(2):165-76. doi: 10.1089/mdr.1997.3.165. Microb Drug Resist. 1997. PMID: 9185145 Review.
-
Two beginnings for a single purpose: the dual-start holins in the regulation of phage lysis.Mol Microbiol. 1996 Aug;21(4):675-82. doi: 10.1046/j.1365-2958.1996.331395.x. Mol Microbiol. 1996. PMID: 8878031 Review.
Cited by
-
Identification of a holin encoded by the Streptomyces aureofaciens phage micro1/6; functional analysis in Escherichia coli system.Folia Microbiol (Praha). 2004;49(6):679-84. doi: 10.1007/BF02931549. Folia Microbiol (Praha). 2004. PMID: 15881403
-
Functional Analysis of the Endopeptidase and Holin From Planktothrix agardhii Cyanophage PaV-LD.Front Microbiol. 2022 Apr 28;13:849492. doi: 10.3389/fmicb.2022.849492. eCollection 2022. Front Microbiol. 2022. PMID: 35572663 Free PMC article.
-
Improving the lethal effect of cpl-7, a pneumococcal phage lysozyme with broad bactericidal activity, by inverting the net charge of its cell wall-binding module.Antimicrob Agents Chemother. 2013 Nov;57(11):5355-65. doi: 10.1128/AAC.01372-13. Epub 2013 Aug 19. Antimicrob Agents Chemother. 2013. PMID: 23959317 Free PMC article.
-
Insights into the structure-function relationships of pneumococcal cell wall lysozymes, LytC and Cpl-1.J Biol Chem. 2008 Oct 17;283(42):28618-28. doi: 10.1074/jbc.M802808200. Epub 2008 Jul 30. J Biol Chem. 2008. PMID: 18667432 Free PMC article.
-
Evidence for a holin-like protein gene fully embedded out of frame in the endolysin gene of Staphylococcus aureus bacteriophage 187.J Bacteriol. 1999 Aug;181(15):4452-60. doi: 10.1128/JB.181.15.4452-4460.1999. J Bacteriol. 1999. PMID: 10419939 Free PMC article.
References
-
- Bläsi U, Halfman G, Lubitz W. Induction of autolysis of Escherichia coli by φX174 gene E product. In: Nombela C, editor. Microbial cell wall synthesis and autolysis. Amsterdam, The Netherlands: Elsevier Science Publishers; 1984. pp. 213–218.
-
- Díaz E, Munthali M, Lünsdorf H, Höltje J-V, Timmis K N. The two-step lysis system of pneumococcal bacteriophage EJ-1 is functional in Gram-negative bacteria: triggering of the major pneumococcal autolysin in Escherichia coli. Mol Microbiol. 1996;19:667–681. - PubMed
-
- García E, Gómez A, Ronda C, Escarmís C, López R. Pneumococcal bacteriophage Cp-1 contains a protein bound to the 5′ termini of its DNA. Virology. 1983;128:92–104. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

