Chemotaxis in Borrelia burgdorferi
- PMID: 9440510
- PMCID: PMC106876
- DOI: 10.1128/JB.180.2.231-235.1998
Chemotaxis in Borrelia burgdorferi
Abstract
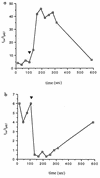
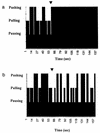
Borrelia burgdorferi is a motile spirochete which has been identified as the causative microorganism in Lyme disease. The physiological functions which govern the motility of this organism have not been elucidated. In this study, we found that motility of B. burgdorferi required an environment similar to interstitial fluid (e.g., pH 7.6 and 0.15 M NaCl). Several methods were used to detect and measure chemotaxis of B. burgdorferi. A number of chemical compounds and mixtures were surveyed for the ability to induce positive and negative chemotaxis of B. burgdorferi. Rabbit serum was found to be an attractant for B. burgdorferi, while ethanol and butanol were found to be repellents. Unlike some free-living spirochetes (e.g., Spirochaeta aurantia), B. burgdorferi did not exhibit any observable chemotaxis to common sugars or amino acids. A method was developed to produce spirochete cells with a self-entangled end. These cells enabled us to study the rotation of a single flagellar bundle in response to chemoattractants or repellents. The study shows that the frequency and duration for pausing of flagella are important for chemotaxis of B. burgdorferi.
Figures

References
-
- Adler J. A method for measuring chemotaxis and use of the method to determine optimum conditions for chemotaxis by Escherichia coli. J Gen Microbiol. 1973;74:77–91. - PubMed
-
- Atsumi T, Maekawa Y, Tokuda H, Imae Y. Amiloride at pH 7.0 inhibits the Na(+)-driven flagellar motors of Vibrio alginolyticus but allows cell growth. FEBS Lett. 1992;314:114–116. - PubMed
-
- Berg H C. How spirochetes may swim. J Theor Biol. 1976;56:269–273. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

