aguA, the gene encoding an extracellular alpha-glucuronidase from Aspergillus tubingensis, is specifically induced on xylose and not on glucuronic acid
- PMID: 9440512
- PMCID: PMC106878
- DOI: 10.1128/JB.180.2.243-249.1998
aguA, the gene encoding an extracellular alpha-glucuronidase from Aspergillus tubingensis, is specifically induced on xylose and not on glucuronic acid
Abstract
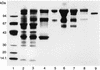
An extracellular alpha-glucuronidase was purified and characterized from a commercial Aspergillus preparation and from culture filtrate of Aspergillus tubingensis. The enzyme has a molecular mass of 107 kDa as determined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and 112 kDa as determined by mass spectrometry, has a determined pI just below 5.2, and is stable at pH 6.0 for prolonged times. The pH optimum for the enzyme is between 4.5 and 6.0, and the temperature optimum is 70 degrees C. The alpha-glucuronidase is active mainly on small substituted xylo-oligomers but is also able to release a small amount of 4-O-methylglucuronic acid from birchwood xylan. The enzyme acts synergistically with endoxylanases and beta-xylosidase in the hydrolysis of xylan. The enzyme is N glycosylated and contains 14 putative N-glycosylation sites. The gene encoding this alpha-glucuronidase (aguA) was cloned from A. tubingensis. It consists of an open reading frame of 2,523 bp and contains no introns. The gene codes for a protein of 841 amino acids, containing a eukaryotic signal sequence of 20 amino acids. The mature protein has a predicted molecular mass of 91,790 Da and a calculated pI of 5.13. Multiple copies of the gene were introduced in A. tubingensis, and expression was studied in a highly overproducing transformant. The aguA gene was expressed on xylose, xylobiose, and xylan, similarly to genes encoding endoxylanases, suggesting a coordinate regulation of expression of xylanases and alpha-glucuronidase. Glucuronic acid did not induce the expression of aguA and also did not modulate the expression on xylose. Addition of glucose prevented expression of aguA on xylan but only reduced the expression on xylose.
Figures
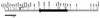


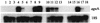
Similar articles
-
Gene cloning and characterization of alpha-glucuronidase of Bacillus stearothermophilus no. 236.Biosci Biotechnol Biochem. 2000 Dec;64(12):2530-7. doi: 10.1271/bbb.64.2530. Biosci Biotechnol Biochem. 2000. PMID: 11210113
-
Regulation of the alpha-glucuronidase-encoding gene ( aguA) from Aspergillus niger.Mol Genet Genomics. 2002 Sep;268(1):96-102. doi: 10.1007/s00438-002-0729-7. Epub 2002 Jul 24. Mol Genet Genomics. 2002. PMID: 12242504
-
Insight into glycoside hydrolases for debranched xylan degradation from extremely thermophilic bacterium Caldicellulosiruptor lactoaceticus.PLoS One. 2014 Sep 3;9(9):e106482. doi: 10.1371/journal.pone.0106482. eCollection 2014. PLoS One. 2014. PMID: 25184498 Free PMC article.
-
Characterization of a recombinant α-glucuronidase from Aspergillus fumigatus.Fungal Biol. 2013 May;117(5):380-7. doi: 10.1016/j.funbio.2013.04.002. Epub 2013 Apr 17. Fungal Biol. 2013. PMID: 23719223
-
[Agua, sodio, potasio y cloruro].World Rev Nutr Diet. 2022;122:110-129. doi: 10.1159/000526501. Epub 2022 Sep 29. World Rev Nutr Diet. 2022. PMID: 36174509 Review. Spanish. No abstract available.
Cited by
-
The membrane-bound alpha-glucuronidase from Pseudomonas cellulosa hydrolyzes 4-O-methyl-D-glucuronoxylooligosaccharides but not 4-O-methyl-D-glucuronoxylan.J Bacteriol. 2002 Sep;184(17):4925-9. doi: 10.1128/JB.184.17.4925-4929.2002. J Bacteriol. 2002. PMID: 12169619 Free PMC article.
-
Overexpression, purification, crystallization and preliminary structural studies of p-coumaric acid decarboxylase from Lactobacillus plantarum.Acta Crystallogr Sect F Struct Biol Cryst Commun. 2007 Apr 1;63(Pt 4):300-3. doi: 10.1107/S1744309107008846. Epub 2007 Mar 12. Acta Crystallogr Sect F Struct Biol Cryst Commun. 2007. PMID: 17401200 Free PMC article.
-
Active fungal GH115 α-glucuronidase produced in Arabidopsis thaliana affects only the UX1-reactive glucuronate decorations on native glucuronoxylans.BMC Biotechnol. 2015 Jun 18;15:56. doi: 10.1186/s12896-015-0154-8. BMC Biotechnol. 2015. PMID: 26084671 Free PMC article.
-
Regulation of the feruloyl esterase (faeA) gene from Aspergillus niger.Appl Environ Microbiol. 1999 Dec;65(12):5500-3. doi: 10.1128/AEM.65.12.5500-5503.1999. Appl Environ Microbiol. 1999. PMID: 10584009 Free PMC article.
-
Aspergillus enzymes involved in degradation of plant cell wall polysaccharides.Microbiol Mol Biol Rev. 2001 Dec;65(4):497-522, table of contents. doi: 10.1128/MMBR.65.4.497-522.2001. Microbiol Mol Biol Rev. 2001. PMID: 11729262 Free PMC article. Review.
References
-
- Benton W D, Davies R W. Screening λgt recombinant clones by hybridization to single plaques in situ. Science. 1977;196:180–182. - PubMed
-
- Bio-Rad Laboratories. Bio-Rad bulletin 1177 EG. Richmond, Calif: Bio-Rad Laboratories; 1984.
-
- Bradford M M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. - PubMed
-
- Bronnenmeier K, Meissner H, Stocker S, Staudenbauer W L. α-d-Glucuronidases from the xylanolytic thermophiles Clostridium stercorarium and Thermoanaerobacterium saccharolyticum. Microbiology. 1995;141:2033–2040. - PubMed
-
- de Graaff L H, van den Broeck H W J, Visser J. Isolation and transformation of the pyruvate kinase gene of Aspergillus nidulans. Curr Genet. 1988;13:315–321. - PubMed
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

