Cloning and characterization of PRA1, a gene encoding a novel pH-regulated antigen of Candida albicans
- PMID: 9440517
- PMCID: PMC106883
- DOI: 10.1128/JB.180.2.282-289.1998
Cloning and characterization of PRA1, a gene encoding a novel pH-regulated antigen of Candida albicans
Abstract
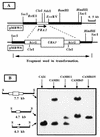
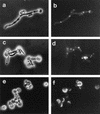
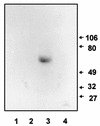
Candida albicans is an opportunistic fungal pathogen of humans. The cell wall of the organism defines the interface between the pathogen and host tissues and is likely to play an essential and pivotal role in the host-pathogen interaction. The components of the cell wall critical to this interaction are undefined. Immunoscreening of a lambda expression library with sera raised against mycelial cell walls of C. albicans was used to identify genes encoding cell surface proteins. One of the positive clones represented a candidal gene that was differentially expressed in response to changes in the pH of the culture medium. Maximal expression occurred at neutral pH, with no expression detected below pH 6.0. On the basis of the expression pattern, the corresponding gene was designated PRA1, for pH-regulated antigen. The protein predicted from the nucleotide sequence was 299 amino acids long with motifs characteristic of secreted glycoproteins. The predicted surface localization and N glycosylation of the protein were directly demonstrated by cell fractionation and immunoblot analysis. Deletion of the gene imparted a temperature-dependent defect in hypha formation, indicating a role in morphogenesis. The PRA1 protein was homologous to surface antigens of Aspergillus spp. which react with serum from aspergillosis patients, suggesting that the PRA1 protein may have a role in the host-parasite interaction during candidal infection.
Figures





Similar articles
-
Candida albicans scavenges host zinc via Pra1 during endothelial invasion.PLoS Pathog. 2012;8(6):e1002777. doi: 10.1371/journal.ppat.1002777. Epub 2012 Jun 28. PLoS Pathog. 2012. PMID: 22761575 Free PMC article.
-
PHR1, a pH-regulated gene of Candida albicans, is required for morphogenesis.Mol Cell Biol. 1995 Feb;15(2):601-13. doi: 10.1128/MCB.15.2.601. Mol Cell Biol. 1995. PMID: 7823929 Free PMC article.
-
Sequence variations and protein expression levels of the two immune evasion proteins Gpm1 and Pra1 influence virulence of clinical Candida albicans isolates.PLoS One. 2015 Feb 18;10(2):e0113192. doi: 10.1371/journal.pone.0113192. eCollection 2015. PLoS One. 2015. PMID: 25692293 Free PMC article.
-
Immune escape of the human facultative pathogenic yeast Candida albicans: the many faces of the Candida Pra1 protein.Int J Med Microbiol. 2011 Jun;301(5):423-30. doi: 10.1016/j.ijmm.2011.04.010. Epub 2011 May 11. Int J Med Microbiol. 2011. PMID: 21565550 Review.
-
High-frequency phenotypic switching in Candida albicans.Trends Genet. 1993 Feb;9(2):61-5. doi: 10.1016/0168-9525(93)90189-O. Trends Genet. 1993. PMID: 8456504 Review.
Cited by
-
Candida albicans scavenges host zinc via Pra1 during endothelial invasion.PLoS Pathog. 2012;8(6):e1002777. doi: 10.1371/journal.ppat.1002777. Epub 2012 Jun 28. PLoS Pathog. 2012. PMID: 22761575 Free PMC article.
-
Effect of environmental pH on morphological development of Candida albicans is mediated via the PacC-related transcription factor encoded by PRR2.J Bacteriol. 1999 Dec;181(24):7524-30. doi: 10.1128/JB.181.24.7524-7530.1999. J Bacteriol. 1999. PMID: 10601210 Free PMC article.
-
Identification of continuous B-cell epitopes on the protein moiety of the 58-kiloDalton cell wall mannoprotein of Candida albicans belonging to a family of immunodominant fungal antigens.Infect Immun. 2001 May;69(5):2909-19. doi: 10.1128/IAI.69.5.2909-2919.2001. Infect Immun. 2001. PMID: 11292706 Free PMC article.
-
The transcription factor Rim101p governs ion tolerance and cell differentiation by direct repression of the regulatory genes NRG1 and SMP1 in Saccharomyces cerevisiae.Mol Cell Biol. 2003 Jan;23(2):677-86. doi: 10.1128/MCB.23.2.677-686.2003. Mol Cell Biol. 2003. PMID: 12509465 Free PMC article.
-
Immunological characterization of Asp f 2, a major allergen from Aspergillus fumigatus associated with allergic bronchopulmonary aspergillosis.Infect Immun. 1998 Nov;66(11):5175-82. doi: 10.1128/IAI.66.11.5175-5182.1998. Infect Immun. 1998. PMID: 9784519 Free PMC article.
References
-
- Altschul S F, Gish W, Miller W, Myers E W, Lipman D J. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. - PubMed
-
- Banerjee B, Kurup V P, Phadnis S, Greenberger P A, Fink J N. Molecular cloning and expression of a recombinant Aspergillus fumigatus protein AspfII with significant immunoglobulin E reactivity in allergic bronchopulmonary aspergillosis. J Lab Clin Med. 1996;127:253–262. - PubMed
-
- Boeke J D, LaCroute F, Fink G R. A positive selection for mutants lacking orotidine-5′-phosphate decarboxylase activity in yeast: 5-fluoro-orotic acid resistance. Mol Gen Genet. 1984;197:345–346. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

