Identification of tropoelastin as a ligand for the 65-kD FK506-binding protein, FKBP65, in the secretory pathway
- PMID: 9442105
- PMCID: PMC2132569
- DOI: 10.1083/jcb.140.2.295
Identification of tropoelastin as a ligand for the 65-kD FK506-binding protein, FKBP65, in the secretory pathway
Abstract
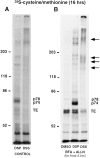
The folding and trafficking of tropoelastin is thought to be mediated by intracellular chaperones, although the identity and role of any tropoelastin chaperone remain to be determined. To identify proteins that are associated with tropoelastin intracellularly, bifunctional chemical cross-linkers were used to covalently stabilize interactions between tropoelastin and associated proteins in the secretory pathway in intact fetal bovine auricular chondrocytes. Immunoprecipitation of tropoelastin from cell lysates after cross-linking and analysis by SDS-PAGE showed the presence of two proteins of approximately 74 kD (p74) and 78 kD (p78) that coimmunoprecipitated with tropoelastin. Microsequencing of peptide fragments from a cyanogen bromide digest of p78 identified this protein as BiP and sequence analysis identified p74 as the peptidyl-prolyl cis-trans isomerase, FKPB65. The appearance of BiP and FKBP65 in the immunoprecipitations could be enhanced by the addition of brefeldin A (BFA) and N-acetyl-leu-leu-norleucinal (ALLN) to the culture medium for the final 4 h of labeling. Tropoelastin accumulates in the fused ER/Golgi compartment in the presence of BFA if its degradation is inhibited by ALLN (Davis, E.C., and R.P. Mecham. 1996. J. Biol. Chem. 271:3787-3794). The use of BFA and other secretion-disrupting agents suggests that the association of tropoelastin with FKBP65 occurs in the ER. Results from this study provide the first identification of a ligand for an FKBP in the secretory pathway and suggest that the prolyl cis-trans isomerase activity of FKBP65 may be important for the proper folding of the proline-rich tropoelastin molecule before secretion.
Figures




Similar articles
-
Selective degradation of accumulated secretory proteins in the endoplasmic reticulum. A possible clearance pathway for abnormal tropoelastin.J Biol Chem. 1996 Feb 16;271(7):3787-94. J Biol Chem. 1996. PMID: 8631995
-
Developmental regulation of FKBP65. An ER-localized extracellular matrix binding-protein.Mol Biol Cell. 2000 Nov;11(11):3925-35. doi: 10.1091/mbc.11.11.3925. Mol Biol Cell. 2000. PMID: 11071917 Free PMC article.
-
Intracellular trafficking of tropoelastin.Matrix Biol. 1998 Aug;17(4):245-54. doi: 10.1016/s0945-053x(98)90078-6. Matrix Biol. 1998. PMID: 9749941
-
Structure-function relationships in the FK506-binding protein (FKBP) family of peptidylprolyl cis-trans isomerases.Biochem J. 1996 Mar 1;314 ( Pt 2)(Pt 2):361-85. Biochem J. 1996. PMID: 8670043 Free PMC article. Review. No abstract available.
-
Immunophilins, ligands, and the control of signal transduction.Harvey Lect. 1995-1996;91:99-114. Harvey Lect. 1995. PMID: 9127988 Review. No abstract available.
Cited by
-
Orchestration of secretory protein folding by ER chaperones.Biochim Biophys Acta. 2013 Nov;1833(11):2410-24. doi: 10.1016/j.bbamcr.2013.03.007. Epub 2013 Mar 15. Biochim Biophys Acta. 2013. PMID: 23507200 Free PMC article. Review.
-
Role of hedgehog signaling in the pathogenesis and therapy of heterotopic ossification.Front Cell Dev Biol. 2024 Sep 19;12:1454058. doi: 10.3389/fcell.2024.1454058. eCollection 2024. Front Cell Dev Biol. 2024. PMID: 39364140 Free PMC article. Review.
-
Loss-of-function mutations in ATP6V0A2 impair vesicular trafficking, tropoelastin secretion and cell survival.Hum Mol Genet. 2009 Jun 15;18(12):2149-65. doi: 10.1093/hmg/ddp148. Epub 2009 Mar 25. Hum Mol Genet. 2009. PMID: 19321599 Free PMC article.
-
Regulation of muscle springiness and hardness: the role of myo-inositol in enhancing fish flesh texture.Food Chem X. 2025 Jul 12;29:102788. doi: 10.1016/j.fochx.2025.102788. eCollection 2025 Jul. Food Chem X. 2025. PMID: 40704186 Free PMC article.
-
Profibrillin-1 maturation by human dermal fibroblasts: proteolytic processing and molecular chaperones.J Cell Biochem. 2003 Oct 15;90(3):641-52. doi: 10.1002/jcb.10657. J Cell Biochem. 2003. PMID: 14523997 Free PMC article.
References
-
- Bächinger HP, Morris NP, Davis JM. Thermal stability and folding of the collagen triple helix and the effects of mutations in osteogenesis imperfecta on the triple helix of type I collagen. Am J Med Genet. 1993;45:152–162. - PubMed
-
- Brown-Augsburger P, Tisdale C, Broekelmann T, Sloan C, Mecham RP. Identification of an elastin cross-linking domain that joins three peptide chains. J Biol Chem. 1995;270:17778–17783. - PubMed
-
- Colley NJ, Baker EK, Stamnes MA, Zuker CS. The cyclophilin homolog ninaA is required in the secretory pathway. Cell. 1991;67:255–263. - PubMed
-
- Coss MC, Winterstein D, Sowder RC, Simek SL. Molecular cloning, DNA sequence analysis, and biochemical characterization of a novel 65-kDa FK506-binding protein (FKBP65) J Biol Chem. 1995;270:29336–29341. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

