Cytoplasmic dynein and dynactin are required for the transport of microtubules into the axon
- PMID: 9442114
- PMCID: PMC2132571
- DOI: 10.1083/jcb.140.2.391
Cytoplasmic dynein and dynactin are required for the transport of microtubules into the axon
Abstract
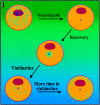
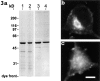
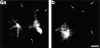
Previous work from our laboratory suggested that microtubules are released from the neuronal centrosome and then transported into the axon (Ahmad, F.J., and P.W. Baas. 1995. J. Cell Sci. 108: 2761-2769). In these studies, cultured sympathetic neurons were treated with nocodazole to depolymerize most of their microtubule polymer, rinsed free of the drug for a few minutes to permit a burst of microtubule assembly from the centrosome, and then exposed to nanomolar levels of vinblastine to suppress further microtubule assembly from occurring. Over time, the microtubules appeared first near the centrosome, then dispersed throughout the cytoplasm, and finally concentrated beneath the periphery of the cell body and within developing axons. In the present study, we microinjected fluorescent tubulin into the neurons at the time of the vinblastine treatment. Fluorescent tubulin was not detected in the microtubules over the time frame of the experiment, confirming that the redistribution of microtubules observed with the experimental regime reflects microtubule transport rather than microtubule assembly. To determine whether cytoplasmic dynein is the motor protein that drives this transport, we experimentally increased the levels of the dynamitin subunit of dynactin within the neurons. Dynactin, a complex of proteins that mediates the interaction of cytoplasmic dynein and its cargo, dissociates under these conditions, resulting in a cessation of all functions of the motor tested to date (Echeverri, C.J., B.M. Paschal, K.T. Vaughan, and R.B. Vallee. 1996. J. Cell Biol. 132: 617-633). In the presence of excess dynamitin, the microtubules did not show the outward progression but instead remained near the centrosome or dispersed throughout the cytoplasm. On the basis of these results, we conclude that cytoplasmic dynein and dynactin are essential for the transport of microtubules from the centrosome into the axon.
Figures


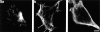


References
-
- Ahmad FJ, Baas PW. Microtubules released from the neuronal centrosome are transported into the axon. J Cell Sci. 1995;108:2761–2769. - PubMed
-
- Ahmad FJ, Joshi HC, Centonze VE, Baas PW. Inhibition of microtubule nucleation at the neuronal centrosome compromises axon growth. Neuron. 1994;12:271–280. - PubMed
-
- Allan V. Motor proteins: a dynamic duo. Curr Biol. 1996;6:630–633. - PubMed
-
- Baas PW. The neuronal centrosome as a generator of microtubules for the axon. Curr Top Dev Biol. 1996;33:281–298. - PubMed
-
- Baas PW. Microtubules and axonal growth. Curr Opin Cell Biol. 1997;9:29–36. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

