Mice that lack thrombospondin 2 display connective tissue abnormalities that are associated with disordered collagen fibrillogenesis, an increased vascular density, and a bleeding diathesis
- PMID: 9442117
- PMCID: PMC2132586
- DOI: 10.1083/jcb.140.2.419
Mice that lack thrombospondin 2 display connective tissue abnormalities that are associated with disordered collagen fibrillogenesis, an increased vascular density, and a bleeding diathesis
Abstract
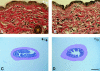
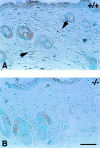
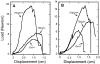
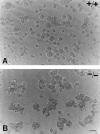
Thrombospondin (TSP) 2, and its close relative TSP1, are extracellular proteins whose functions are complex, poorly understood, and controversial. In an attempt to determine the function of TSP2, we disrupted the Thbs2 gene by homologous recombination in embryonic stem cells, and generated TSP2-null mice by blastocyst injection and appropriate breeding of mutant animals. Thbs2-/- mice were produced with the expected Mendelian frequency, appeared overtly normal, and were fertile. However, on closer examination, these mice displayed a wide variety of abnormalities. Collagen fiber patterns in skin were disordered, and abnormally large fibrils with irregular contours were observed by electron microscopy in both skin and tendon. As a functional correlate of these findings, the skin was fragile and had reduced tensile strength, and the tail was unusually flexible. Mutant skin fibroblasts were defective in attachment to a substratum. An increase in total density and in cortical thickness of long bones was documented by histology and quantitative computer tomography. Mutant mice also manifested an abnormal bleeding time, and histologic surveys of mouse tissues, stained with an antibody to von Willebrand factor, showed a significant increase in blood vessels. The basis for the unusual phenotype of the TSP2-null mouse could derive from the structural role that TSP2 might play in collagen fibrillogenesis in skin and tendon. However, it seems likely that some of the diverse manifestations of this genetic disorder result from the ability of TSP2 to modulate the cell surface properties of mesenchymal cells, and thus, to affect cell functions such as adhesion and migration.
Figures







References
-
- Adams JC. Formation of stable microspikes containing actin and the 55 kda actin bundling protein, fascin, is a consequence of cell adhesion to thrombospondin-1: implications for the anti-adhesive activities of thrombospondin-1. J Cell Sci. 1995;108:1977–1990. - PubMed
-
- Adams JC, Lawler J. Diverse mechanisms for cell attachment to platelet thrombospondin. J Cell Sci. 1993;104:1061–1071. - PubMed
-
- Andrikopoulos K, Liu X, Keene DR, Jaenisch R, Ramirez F. Targeted mutation in the Col5α2 gene reveals a regulatory role for type V collagen during matrix assembly. Nat Genet. 1995;9:31–36. - PubMed
-
- Aumailley M, Mann K, von der Mark H, Timpl R. Call attachment properties of collagen type VI and Arg-Gly-Asp dependent binding to its α2(VI) and α3(VI) chains. Exp Cell Res. 1989;181:463–474. - PubMed
-
- Barabino GA, Wise RJ, Woodbury VA, Zhang BB, Bridges KA, Hebbel RP, Lawler J, Ewenstein BM. Inhibition of sickle erythrocyte adhesion to immobilized thrombospondin by von willebrand factor under dynamic flow conditions. Blood. 1997;89:2560–2567. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

