The two-component signaling pathway of bacterial chemotaxis: a molecular view of signal transduction by receptors, kinases, and adaptation enzymes
- PMID: 9442881
- PMCID: PMC2899694
- DOI: 10.1146/annurev.cellbio.13.1.457
The two-component signaling pathway of bacterial chemotaxis: a molecular view of signal transduction by receptors, kinases, and adaptation enzymes
Abstract
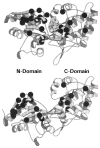
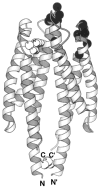
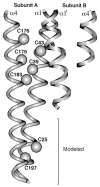
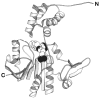
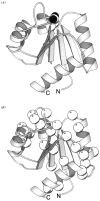
The chemosensory pathway of bacterial chemotaxis has become a paradigm for the two-component superfamily of receptor-regulated phosphorylation pathways. This simple pathway illustrates many of the fundamental principles and unanswered questions in the field of signaling biology. A molecular description of pathway function has progressed rapidly because it is accessible to diverse structural, biochemical, and genetic approaches. As a result, structures are emerging for most of the pathway elements, biochemical studies are elucidating the mechanisms of key signaling events, and genetic methods are revealing the intermolecular interactions that transmit information between components. Recent advances include (a) the first molecular picture of a conformational transmembrane signal in a cell surface receptor, (b) four new structures of kinase domains and adaptation enzymes, and (c) significant new insights into the mechanisms of receptor-mediated kinase regulation, receptor adaptation, and the phospho-activation of signaling proteins. Overall, the chemosensory pathway and the propulsion system it regulates provide an ideal system in which to probe molecular principles underlying complex cellular signaling and behavior.
Figures




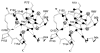




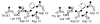

References
-
- Ames P, Chen J, Wolff A, Parkinson JS. Structure-function studies of bacterial chemosensors. Cold Spring Harbor Symp Quant Biol. 1988;53:59–66. - PubMed
-
- Ames P, Yu AL, Parkinson JS. Methylation segments are not required for chemotactic signaling by cytoplasmic fragments of Tsr, the methyl-accepting serine chemoreceptor of Escherichia coli. Mol Microbiol. 1996;19:737–46. - PubMed
-
- Appleby JL, Parkinson JS, Bourret RB. Signal transduction via the multistep phosphorelay. Not necessarily a road less traveled. Cell. 1996;86:845–48. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

