Production and characterization of improved adenovirus vectors with the E1, E2b, and E3 genes deleted
- PMID: 9444984
- PMCID: PMC124562
- DOI: 10.1128/JVI.72.2.926-933.1998
Production and characterization of improved adenovirus vectors with the E1, E2b, and E3 genes deleted
Abstract
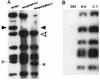
Adenovirus (Ad)-based vectors have great potential for use in the gene therapy of multiple diseases, both genetic and nongenetic. While capable of transducing both dividing and quiescent cells efficiently, Ad vectors have been limited by a number of problems. Most Ad vectors are engineered such that a transgene replaces the Ad E1a, E1b, and E3 genes; subsequently the replication-defective vector can be propagated only in human 293 cells that supply the deleted E1 gene functions in trans. Unfortunately, the use of high titers of E1-deleted vectors has been repeatedly demonstrated to result in low-level expression of viral genes still resident in the vector. In addition, the generation of replication-competent Ad (RCA) by recombination events with the E1 sequences residing in 293 cells further limits the usefulness of E1-deleted Ad vectors. We addressed these problems by isolating new Ad vectors deleted for the E1, E3, and the E2b gene functions. The new vectors can be readily grown to high titers and have several improvements, including an increased carrying capacity and a theoretically decreased risk for generating RCA. We have also demonstrated that the further block to Ad vector replication afforded by the deletion of both the E1 and E2b genes significantly diminished Ad late gene expression in comparison to a conventional E1-deleted vector, without destabilization of the modified vector genome. The results suggested that these modified vectors may be very useful both for in vitro and in vivo gene therapy applications.
Figures



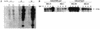


Similar articles
-
Optimization of vaccine responses with an E1, E2b and E3-deleted Ad5 vector circumvents pre-existing anti-vector immunity.Cancer Gene Ther. 2009 Sep;16(9):673-82. doi: 10.1038/cgt.2009.17. Epub 2009 Feb 20. Cancer Gene Ther. 2009. PMID: 19229288 Free PMC article.
-
Generation of an adenovirus vector lacking E1, e2a, E3, and all of E4 except open reading frame 3.J Virol. 1999 Jul;73(7):6048-55. doi: 10.1128/JVI.73.7.6048-6055.1999. J Virol. 1999. PMID: 10364357 Free PMC article.
-
Construction and characterization of E1-minus replication-defective adenovirus vectors that express E3 proteins from the E1 region.Virology. 2002 Sep 15;301(1):99-108. doi: 10.1006/viro.2002.1580. Virology. 2002. PMID: 12359450
-
Adenovirus vectors deleted for genes essential for viral DNA replication.Front Biosci. 2005 May 1;10:1146-55. doi: 10.2741/1607. Front Biosci. 2005. PMID: 15769613 Review.
-
[Development of a novel adenovirus vector exhibiting microRNA-mediated suppression of the leaky expression of adenovirus genes].Yakugaku Zasshi. 2012;132(12):1407-12. doi: 10.1248/yakushi.12-00235-5. Yakugaku Zasshi. 2012. PMID: 23208048 Review. Japanese.
Cited by
-
The function and molecular identity of inward rectifier channels in vestibular hair cells of the mouse inner ear.J Neurophysiol. 2012 Jul;108(1):175-86. doi: 10.1152/jn.00098.2012. Epub 2012 Apr 11. J Neurophysiol. 2012. PMID: 22496522 Free PMC article.
-
Anti-tumor immunotherapy despite immunity to adenovirus using a novel adenoviral vector Ad5 [E1-, E2b-]-CEA.Cancer Immunol Immunother. 2010 Jul;59(7):1131-5. doi: 10.1007/s00262-010-0847-8. Epub 2010 Apr 2. Cancer Immunol Immunother. 2010. PMID: 20361185 Free PMC article.
-
CR1/2 is an important suppressor of Adenovirus-induced innate immune responses and is required for induction of neutralizing antibodies.Gene Ther. 2009 Oct;16(10):1245-59. doi: 10.1038/gt.2009.77. Epub 2009 Jun 25. Gene Ther. 2009. PMID: 19554032 Free PMC article.
-
Mechanotransduction in mouse inner ear hair cells requires transmembrane channel-like genes.J Clin Invest. 2011 Dec;121(12):4796-809. doi: 10.1172/JCI60405. Epub 2011 Nov 21. J Clin Invest. 2011. PMID: 22105175 Free PMC article.
-
TRIF, and TRIF-interacting TLRs differentially modulate several adenovirus vector-induced immune responses.J Innate Immun. 2009;1(4):376-88. doi: 10.1159/000207194. Epub 2009 Mar 4. J Innate Immun. 2009. PMID: 20375595 Free PMC article.
References
-
- Amalfitano A, Chamberlain J S. Isolation and characterization of packaging cell lines that coexpress the adenovirus DNA polymerase and preterminal proteins: implications for gene therapy. Gene Ther. 1997;4:258–263. - PubMed
-
- Askari F K, Hitomi Y, Mao M, Wilson J M. Complete correction of hyperbilirubinemia in the Gunn rat model of Crigler-Najjar syndrome type I following transient in vivo adenovirus-mediated expression of human bilirubin UDP-glucuronosyltransferase. Gene Ther. 1996;3:381–388. - PubMed
-
- Barr D, Tubb J, Ferguson D, Scarla A, Lieber A, Wilson C, Perkins J, Kay M A. Strain related variations in adenovirally mediated transgene expression from mouse hepatocytes in vivo: comparisons between immunocompetent and immunodeficient inbred strains. Gene Ther. 1995;2:151–155. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

