DNA immunization against herpes simplex virus: enhanced efficacy using a Sindbis virus-based vector
- PMID: 9444987
- PMCID: PMC124565
- DOI: 10.1128/JVI.72.2.950-958.1998
DNA immunization against herpes simplex virus: enhanced efficacy using a Sindbis virus-based vector
Abstract
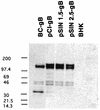
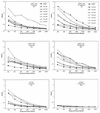
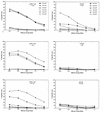
Previously we reported the development of a plasmid DNA expression vector system derived from Sindbis virus (T. W. Dubensky, Jr., et al., J. Virol. 70:508-519, 1996). In vitro, such vectors exhibit high-level heterologous gene expression via self-amplifying cytoplasmic RNA replication. In the present study, we demonstrated the in vivo efficacy of the Sindbis virus-based pSIN vectors as DNA vaccines. A single intramuscular immunization of BALB/c mice with pSIN vectors expressing the glycoprotein B of herpes simplex virus type 1 induced a broad spectrum of immune responses, including virus-specific antibodies, cytotoxic T cells, and protection from lethal virus challenge in two different murine models. In addition, dosing studies demonstrated that the pSIN vectors were superior to a conventional plasmid DNA vector in the induction of all immune parameters tested. In general, 100- to 1,000-fold-lower doses of pSIN were needed to induce the same level of responsiveness as that achieved with the conventional plasmid DNA vector. In some instances, significant immune responses were induced with a single dose of pSIN as low as 10 ng/mouse. These results indicate the potential usefulness of alphavirus-based vectors for DNA immunization in general and more specifically as a herpes simplex virus vaccine.
Figures


Similar articles
-
Vaccination of mice with herpes simplex virus type 1 glycoprotein D DNA produces low levels of protection against lethal HSV-1 challenge.Antiviral Res. 1995 Oct;28(2):147-57. doi: 10.1016/0166-3542(95)00045-n. Antiviral Res. 1995. PMID: 8585768
-
Induction of mucosal immunity against herpes simplex virus by plasmid DNA immunization.J Virol. 1997 Apr;71(4):3138-45. doi: 10.1128/JVI.71.4.3138-3145.1997. J Virol. 1997. PMID: 9060677 Free PMC article.
-
Sindbis virus DNA-based expression vectors: utility for in vitro and in vivo gene transfer.J Virol. 1996 Jan;70(1):508-19. doi: 10.1128/JVI.70.1.508-519.1996. J Virol. 1996. PMID: 8523564 Free PMC article.
-
Adenovirus vectors as potential vaccines against herpes simplex virus.Rev Infect Dis. 1991 Nov-Dec;13 Suppl 11:S912-6. doi: 10.1093/clind/13.supplement_11.s912. Rev Infect Dis. 1991. PMID: 1664127 Review.
-
Live vaccinia virus recombinants expressing herpes simplex virus genes.Rev Infect Dis. 1991 Nov-Dec;13 Suppl 11:S898-903. doi: 10.1093/clind/13.supplement_11.s898. Rev Infect Dis. 1991. PMID: 1664124 Review.
Cited by
-
Infection of human dendritic cells by a sindbis virus replicon vector is determined by a single amino acid substitution in the E2 glycoprotein.J Virol. 2000 Dec;74(24):11849-57. doi: 10.1128/jvi.74.24.11849-11857.2000. J Virol. 2000. PMID: 11090185 Free PMC article.
-
Stable alphavirus packaging cell lines for Sindbis virus and Semliki Forest virus-derived vectors.Proc Natl Acad Sci U S A. 1999 Apr 13;96(8):4598-603. doi: 10.1073/pnas.96.8.4598. Proc Natl Acad Sci U S A. 1999. PMID: 10200308 Free PMC article.
-
Alphavirus-based DNA vaccine breaks immunological tolerance by activating innate antiviral pathways.Nat Med. 2003 Jan;9(1):33-9. doi: 10.1038/nm813. Epub 2002 Dec 23. Nat Med. 2003. PMID: 12496961 Free PMC article.
-
Alphavirus replicon DNA expressing HIV antigens is an excellent prime for boosting with recombinant modified vaccinia Ankara (MVA) or with HIV gp140 protein antigen.PLoS One. 2015 Feb 2;10(2):e0117042. doi: 10.1371/journal.pone.0117042. eCollection 2015. PLoS One. 2015. PMID: 25643354 Free PMC article.
-
Role of innate signalling pathways in the immunogenicity of alphaviral replicon-based vaccines.Virol J. 2011 Jan 24;8:36. doi: 10.1186/1743-422X-8-36. Virol J. 2011. PMID: 21261958 Free PMC article.
References
-
- Banks T A, Jenkins F J, Kanangat S, Nair S, Dasgupta S, Foster C M, Rouse B T. Vaccination with the immediate-early protein ICP47 of herpes simplex virus type 1 (HSV-1) induces virus-specific lymphoproliferation, but fails to protect against lethal challenge. Virology. 1994;200:236–245. - PubMed
-
- Bourne N, Milligan G N, Schleiss M R, Bernstein D I, Stanberry L R. DNA immunization confers protective immunity on mice challenged intravaginally with herpes simplex virus type 2. Vaccine. 1996;14:1230–1234. - PubMed
-
- Bourne N, Stanberry L R, Bernstein D I, Lew D. DNA immunization against experimental genital herpes simplex virus infection. J Infect Dis. 1996;173:800–807. - PubMed
-
- Boyer J D, Ugen K E, Wang B, Agadjanyan M, Gilbert L, Bagarazzi M L, Chattergoon M, Frost P, Javadian A, Williams W V, Refaeli Y, Ciccarelli R B, McCallus D, Coney L, Weiner D B. Protection of chimpanzees from high-dose heterologous HIV-1 challenge by DNA vaccination. Nat Med. 1997;3:526–532. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

